
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Chapter 8, Problem 65P
Interpretation Introduction
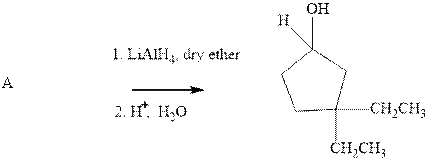
Interpretation:The best structure for substrate A should be chosen.
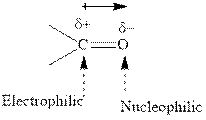
Concept introduction:The carbonyl bond is polar with partial postive charge on carbon and partial negative charge on oxygen as illustrated below.
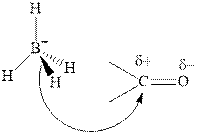
Thus it can undergo hydride addition at carbon and proton addition at oxygen. Certain reagents that are useful for such hydride addition at carbonyl carbon include
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Formulate the products obtained by reacting p-toluidine with a sulfonate mixture. Indicate the majority if necessary.
Consider this organic reaction:
OH
Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant
rate, check the box under the drawing area instead.
Click and drag to start drawing a structure.
x
0:
の
C
Explain the reasons for a compound's greater or lesser reactivity toward electrophilic aromatic substitution. Give reasons.
Chapter 8 Solutions
Organic Chemistry: Structure and Function
Ch. 8.1 - Prob. 8.1ECh. 8.1 - Prob. 8.2ECh. 8.3 - Prob. 8.4TIYCh. 8.3 - Prob. 8.5ECh. 8.3 - Prob. 8.6ECh. 8.4 - Prob. 8.7ECh. 8.5 - Prob. 8.8ECh. 8.5 - Prob. 8.9ECh. 8.5 - Prob. 8.10ECh. 8.5 - Prob. 8.11E
Ch. 8.5 - Prob. 8.12ECh. 8.6 - Prob. 8.14TIYCh. 8.7 - Prob. 8.15ECh. 8.7 - Prob. 8.16ECh. 8.8 - Prob. 8.18TIYCh. 8.8 - Prob. 8.19ECh. 8.8 - Prob. 8.21TIYCh. 8 - Prob. 24PCh. 8 - Prob. 25PCh. 8 - Prob. 26PCh. 8 - Prob. 27PCh. 8 - Prob. 28PCh. 8 - Prob. 29PCh. 8 - Prob. 30PCh. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - Prob. 34PCh. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - Prob. 38PCh. 8 - Prob. 39PCh. 8 - Prob. 40PCh. 8 - Prob. 41PCh. 8 - Prob. 42PCh. 8 - Prob. 43PCh. 8 - Prob. 44PCh. 8 - Prob. 45PCh. 8 - Prob. 46PCh. 8 - Prob. 47PCh. 8 - Prob. 48PCh. 8 - Prob. 49PCh. 8 - Prob. 50PCh. 8 - Prob. 51PCh. 8 - Prob. 52PCh. 8 - Prob. 53PCh. 8 - Prob. 54PCh. 8 - Prob. 55PCh. 8 - Prob. 56PCh. 8 - Prob. 57PCh. 8 - Prob. 58PCh. 8 - Prob. 59PCh. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - Prob. 64PCh. 8 - Prob. 65PCh. 8 - Prob. 66P
Knowledge Booster
Similar questions
- Draw the products of a reaction of the following alkyle chloride, shown below in the 3D ball and stick model with NaSCH3. Ignore inorganic byproducts. In the figure, a gray ball indicates a carbon atom a white ball indicates a hydrogen atom anda agreen ball indicated a chlorine atomarrow_forwardDraw the most stable cations formed in the mass spectrometer by a deavage of the following compound Draw the most stable cations formed in the mass spectrometer by a cleavage of the following compound онarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting anand product sytucutrs, draw the curved electron-pusing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bind-making stepsarrow_forward
- Draw the major elimination and substitution products formed in this reavtion. Use a dash or wedge bond to indicatr the stereochemistry of substituents on assymetric centers, wheere applicable. Ignore any inorganic byproducts.arrow_forwardDraw the two possible products produced in this E2 elimination. Ignore any inorganic byproductsarrow_forwardDraw the major products of this SN1 reaction. Ignore any inorganic byproducts.arrow_forward
- Draw the major elimination and substitution products formed in this reaction. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, wehre applicable. Ignore and inorganic byproducts.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Drawing Arrows THE Problem 33 of 35 N. C:0 Na + Submit Drag To Pan +arrow_forwardDraw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore and inorganic byproducts.arrow_forward
- Draw the major producrs of this SN1 reaction. Ignore any inorganic byproducts. Use a dash or wedge bond to indicate the sereochemistry of substituents on asymmetric centers where appllicable.arrow_forward5) Oxaloacetic Acid is an important intermediate in the biosynthesis of citric acid. Synthesize oxaloacetic acid using a mixed Claisen Condensation reaction with two different esters and a sodium ethoxide base. Give your answer as a scheme Hint 1: Your final acid product is producing using a decarboxylation reaction. Hint 2: Look up the structure of oxalic acid. HO all OH oxaloacetic acidarrow_forward20. The Brusselator. This hypothetical system was first proposed by a group work- ing in Brussels [see Prigogine and Lefever (1968)] in connection with spatially nonuniform chemical patterns. Because certain steps involve trimolecular reac tions, it is not a model of any real chemical system but rather a prototype that has been studied extensively. The reaction steps are A-X. B+X-Y+D. 2X+ Y-3X, X-E. 305 It is assumed that concentrations of A, B, D, and E are kept artificially con stant so that only X and Y vary with time. (a) Show that if all rate constants are chosen appropriately, the equations de scribing a Brusselator are: dt A-(B+ 1)x + x²y, dy =Bx-x²y. diarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning