
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 8, Problem 53P
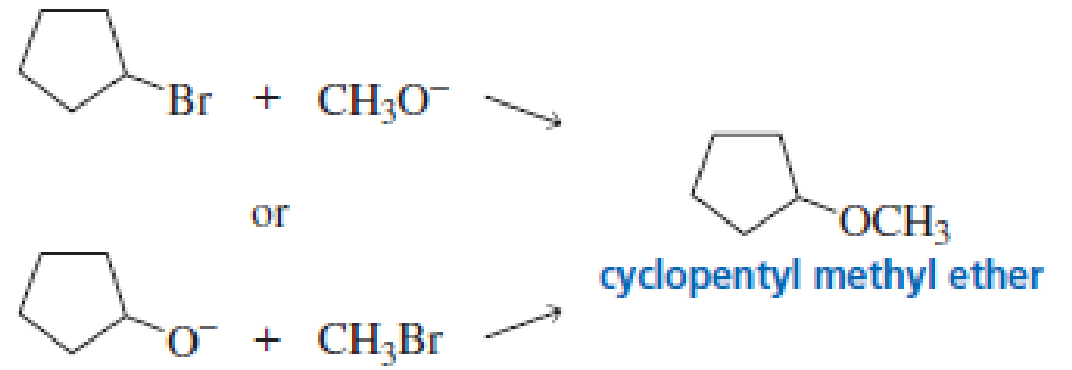
An ether can be prepared by an SN2 reaction of an
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1-bromo-5-chloropentane
LDA
1 eq
a) Hg(OAc)2
b) NaBH
NaOH (1 eq)
CHOO
Note this product has a ring. The nucleophile
and the alkene in this reaction are both in the
same molecule! Also remember that alcohols
react much like water!
CH10O
Note this product has both alcohol
and alkene functional groups
Br
Br
CH₂ONa (1 eq)
+
S2
OCH
Show how each of the following compounds can be prepared from the given starting material. In each case, you will need to use a protecting group.
b) The Wolf-Kishner reduction is a reaction used in Organic Chemistry to convert
carbonyl functionalities into methylene group. The reaction was used to convert
an aldehyde or ketone to an alkane using hydrazine, base and thermal conditions.
The mechanism begins with the attack of hydrazine of the aldehyde or ketone.
Stage 1: The reaction of aldehyde/ketone with hydrazine to produce hydrazine
Stage 2: Reaction with the base and heat to convert hydrozone to alkane
Write the mechanism of the reaction.
Chapter 8 Solutions
Essential Organic Chemistry, Global Edition
Ch. 8.1 - Prob. 2PCh. 8.1 - Does increasing the energy barrier for an SN2...Ch. 8.1 - Arrange the following alkyl bromides in order from...Ch. 8.2 - Prob. 7PCh. 8.2 - Which reaction in each of the following pairs...Ch. 8.2 - Prob. 9PCh. 8.2 - Prob. 11PCh. 8.3 - Draw the substitution products that will be formed...Ch. 8.4 - Arrange the following alkyl halides in order from...Ch. 8.5 - Prob. 14P
Ch. 8.5 - Which of the following reactions will go faster if...Ch. 8.6 - After a proton is removed from the OH group, which...Ch. 8.6 - Draw the product of each of the following...Ch. 8.9 - Prob. 20PCh. 8.9 - Prob. 21PCh. 8.11 - Why do the SN1/E1 reactions of tertiary alkyl...Ch. 8.11 - Prob. 23PCh. 8.11 - Prob. 24PCh. 8.12 - Prob. 25PCh. 8.12 - Prob. 26PCh. 8.12 - Prob. 27PCh. 8.12 - a In which solvent would tert-butyl bromide...Ch. 8.13 - What would be the best way to prepare the...Ch. 8 - Methoxychlor is an insecticide that was intended...Ch. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - Prob. 34PCh. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Explain how the following changes would affect the...Ch. 8 - Prob. 38PCh. 8 - Draw the major product obtained when each of the...Ch. 8 - Which alkyl halide in Problem 39 can undergo an El...Ch. 8 - Prob. 42PCh. 8 - Prob. 43PCh. 8 - Prob. 44PCh. 8 - Prob. 45PCh. 8 - Starting with bromocyclohexane, how could the...Ch. 8 - Prob. 48PCh. 8 - Fill in the blanks in the following chemical...Ch. 8 - For each of the following alkyl halides, indicate...Ch. 8 - Prob. 51PCh. 8 - a. Explain why 1-bromo-2,2-dimethylpropane has...Ch. 8 - An ether can be prepared by an SN2 reaction of an...Ch. 8 - Give two sets of reactants (each set including an...Ch. 8 - Show how the following compounds could be...Ch. 8 - Prob. 56PCh. 8 - Prob. 57PCh. 8 - Draw the structures of the products obtained from...Ch. 8 - cis-4-Bromocyclohexanol and...Ch. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - In which solventethanol or diethyl etherwould the...Ch. 8 - The pKa of acetic acid in water is 4.76. What...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the role of phosphoric acid in the synthesis of cyclohexene? it is an antioxidant that prevents free radical side reactions it is a safe, non-toxic solvent it lowers the boiling point of the reaction mixture (a colligative property of adding phosphoric acid to water) it protonates the hydroxyl of cyclohexanol to make it a better leaving grouparrow_forwarduo 9:0A Asiacell I. 02:48 المتبقي متعد د الخيارات Which alkyl halide would react the fastest under substitution conditions with HO-? 2-bromo-2-methylpentane O 2-bromo-3-methylpentane 1-bromo-2,2-dimethylpentane 2-bromopentane 1-bromo-4-methylpentane O 1 of 5arrow_forwardb) Synthesize the following product starting with phenol (hydroxybenzene). NO₂ OH HO. phenolarrow_forward
- 1. Predict the product, and propose a mechanism for the following nucleophilic addition to aldehyde or ketone. Are the products different from one another? Why do the reactions proceed via different mechanisms? Note the charges on the intermediates of acid and base catalyzed reactions. Oxygen nucleophile in acidic condition H2O, [H+] Oxygen nucleophile in basic condition NaOHarrow_forward4. Aldehydes and ketones can be "protected" as acetals and ketals. Complete the following outlined reactions, showing how the protection is accomplished, and what results after deprotection. Br MgBr 1) Br 2) H3O* deprotection herearrow_forwardDraw the alkyl bromide and the nucleophilearrow_forward
- 4. Which of the following reactions can be used to prepare 11-diphenyl-1-propanol? OH I. II. III. CH₂CH₂C снене-ось CH₂CH₂C -OCH3 PO 1. 2. H₂00 1. ? 2. H30Ⓡ MgBr MgBr 1. CH3CH₂ MgBr 2. H30arrow_forward4. A CHEM 245 student wants to synthesize some ethylene glycol to use as antifreeze in his radiator this winter. He proposes the following reaction to his instructor, who quickly explains that this reaction won't work as proposed due to the student's choice of reagent. 1) NaH 2) H20 OH Но a) Why can't sodium hydride (NaH) be used as the nucleophile in the reaction above? b) Propose an alternate reagent that COULD be used with the ethylene oxide to successfully give the desired ethylene glycol product.arrow_forwardhelparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY