
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 8, Problem 49P
Fill in the blanks in the following chemical equations:
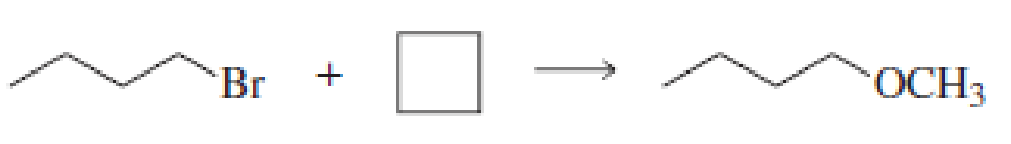
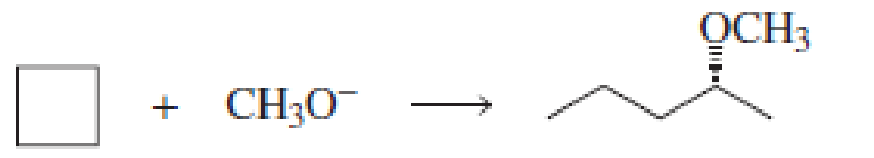
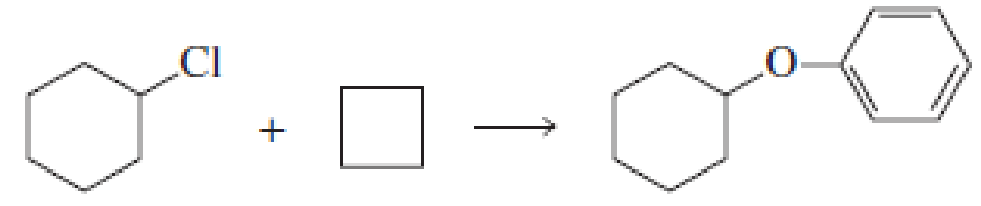
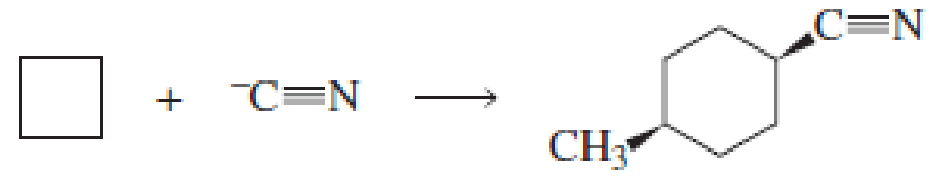
Expert Solution & Answer
Learn your wayIncludes step-by-step video

schedule06:13
Students have asked these similar questions
Decide whether these proposed Lewis structures are reasonable.
proposed Lewis structure
Is the proposed Lewis structure reasonable?
Yes.
:0:
Cl C C1:
0=0:
: 0 :
: 0 :
H C N
No, it has the wrong number of valence electrons.
The correct number is: ☐
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:* ☐
Yes.
No, it has the wrong number of valence electrons.
The correct number is: ☐
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:*
Yes.
☐
No, it has the wrong number of valence electrons.
The correct number is: ☐
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:* |
* If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many
times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".
Draw the Lewis structure for the polyatomic trisulfide
anion. Be sure to include all resonance structures that satisfy the octet rule.
с
[ ] -
G
1. Calculate the accurate monoisotopic mass (using all 1H, 12C, 14N, 160 and 35CI) for your product using the table in
your lab manual. Don't include the Cl, since you should only have [M+H]*. Compare this to the value you see on
the LC-MS printout. How much different are they?
2. There are four isotopic peaks for the [M+H]* ion at m/z 240, 241, 242 and 243. For one point of extra credit,
explain what each of these is and why they are present.
3. There is a fragment ion at m/z 184. For one point of extra credit, identify this fragment and confirm by
calculating the accurate monoisotopic mass.
4. The UV spectrum is also at the bottom of your printout. For one point of extra credit, look up the UV spectrum
of bupropion on Google Images and compare to your spectrum. Do they match? Cite your source.
5. For most of you, there will be a second chromatographic peak whose m/z is 74 (to a round number). For one
point of extra credit, see if you can identify this molecule as well and confirm by…
Chapter 8 Solutions
Essential Organic Chemistry, Global Edition
Ch. 8.1 - Prob. 2PCh. 8.1 - Does increasing the energy barrier for an SN2...Ch. 8.1 - Arrange the following alkyl bromides in order from...Ch. 8.2 - Prob. 7PCh. 8.2 - Which reaction in each of the following pairs...Ch. 8.2 - Prob. 9PCh. 8.2 - Prob. 11PCh. 8.3 - Draw the substitution products that will be formed...Ch. 8.4 - Arrange the following alkyl halides in order from...Ch. 8.5 - Prob. 14P
Ch. 8.5 - Which of the following reactions will go faster if...Ch. 8.6 - After a proton is removed from the OH group, which...Ch. 8.6 - Draw the product of each of the following...Ch. 8.9 - Prob. 20PCh. 8.9 - Prob. 21PCh. 8.11 - Why do the SN1/E1 reactions of tertiary alkyl...Ch. 8.11 - Prob. 23PCh. 8.11 - Prob. 24PCh. 8.12 - Prob. 25PCh. 8.12 - Prob. 26PCh. 8.12 - Prob. 27PCh. 8.12 - a In which solvent would tert-butyl bromide...Ch. 8.13 - What would be the best way to prepare the...Ch. 8 - Methoxychlor is an insecticide that was intended...Ch. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - Prob. 34PCh. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Explain how the following changes would affect the...Ch. 8 - Prob. 38PCh. 8 - Draw the major product obtained when each of the...Ch. 8 - Which alkyl halide in Problem 39 can undergo an El...Ch. 8 - Prob. 42PCh. 8 - Prob. 43PCh. 8 - Prob. 44PCh. 8 - Prob. 45PCh. 8 - Starting with bromocyclohexane, how could the...Ch. 8 - Prob. 48PCh. 8 - Fill in the blanks in the following chemical...Ch. 8 - For each of the following alkyl halides, indicate...Ch. 8 - Prob. 51PCh. 8 - a. Explain why 1-bromo-2,2-dimethylpropane has...Ch. 8 - An ether can be prepared by an SN2 reaction of an...Ch. 8 - Give two sets of reactants (each set including an...Ch. 8 - Show how the following compounds could be...Ch. 8 - Prob. 56PCh. 8 - Prob. 57PCh. 8 - Draw the structures of the products obtained from...Ch. 8 - cis-4-Bromocyclohexanol and...Ch. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - In which solventethanol or diethyl etherwould the...Ch. 8 - The pKa of acetic acid in water is 4.76. What...
Additional Science Textbook Solutions
Find more solutions based on key concepts
53. This reaction was monitored as a function of time:
A plot of In[A] versus time yields a straight ...
Chemistry: Structure and Properties (2nd Edition)
What is the difference between cellular respiration and external respiration?
Human Physiology: An Integrated Approach (8th Edition)
The number of named species is about ________, but the actual number of species on Earth is estimated to be abo...
Biology: Life on Earth with Physiology (11th Edition)
In the light reactions, what is the initial electron donor? Where do the electrons finally end up?
Campbell Biology (11th Edition)
APPLY 1.2 Express the following quantities in scientific notation
using fundamental SI units of mass and lengt...
Chemistry (7th Edition)
Q1. Which wavelength of light has the highest frequency?
a) 10 nm
b) 10 mm
c) 1 nm
d) 1 mm
Chemistry: A Molecular Approach (4th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please draw, not just describe!arrow_forwardcan you draw each step on a piece of a paper please this is very confusing to mearrow_forward> Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? esc ? A O O •If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. olo 18 Ar Explanation Check BB Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forward
- Name the structurearrow_forward> For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forwardHow to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forward
- Predict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forwardDraw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) Δ ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Explanation Check X ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forward
- For the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges. :ÖH Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.arrow_forwardUsing the table of Reactants and Products provided in the Hints section, provide the major product (with the correct stereochemistry when applicable) for questions below by selecting the letter that corresponds to the exact chemical structures for the possible product. OH conc Hydrochloric acid 40°C Temp A/arrow_forwardUsing arrows to designate the flow of electrons, complete the reaction below and provide a detailed mechanism for the formation of the product OH conc Hydrochloric acid 40°C Temp All chemical structures should be hand drawn on a piece of paper Paragraph BI UAE +varrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
SAR of Anticancer(Antineoplastic) Drug/ Alkylating agents/ Nitrogen Mustard; Author: Pharmacy Lectures;https://www.youtube.com/watch?v=zrzyK3LhUXs;License: Standard YouTube License, CC-BY