
Concept explainers
Limonene is a compound found in orange oil and lemon oil. When limonene is treated with excess hydrogen and a platinum catalyst, the product of the reaction is 1-isopropyl-4-methylcyclohexane. When limonene is treated with ozone and then with dimethyl sulfide
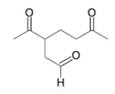
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Campbell Biology (11th Edition)
Campbell Essential Biology (7th Edition)
Microbiology with Diseases by Body System (5th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Living By Chemistry: First Edition Textbook
Organic Chemistry (8th Edition)
- What is the differences between DNA and RNA for the following: - structure - function - type What is the meaning of: - replication - transcription - translation show the base pair connection(hydrogen bond) in DNA and RNAarrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
- > aw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 1. Eto 1. EtO¯ H3O+ 1 2 2. PrBr 2. PrBr Δ You can draw the three structures in any arrangement you like. 3 Click and drag to start drawing a structure. Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacarrow_forwardThere are various factors that affect an equilibrium. Give 3 of these factors and explain using examples andequations how an equilibrium is affected by these factors. Please remember that this is a communication question so that you are communicating your understanding of the factors that affect and equilibrium.arrow_forwardEEZE LETCHUP ID Draw the most likely conjugate base resulting from this acid-base reaction. Include all lone pairs. Ignore inorganic byproducts. Drawing く NaOCH2CH3 :0: :0: 狗arrow_forward
- Answerarrow_forward2. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. CH3 Ph OEt هد Ph CH3 Hint: the species on the left is an ynolate, which behaves a lot like an enolate.arrow_forwardb. CH3 H3C CH3 CH3 H3C an unexpected product, containing a single 9- membered ring the expected product, containing two fused rings H3C-I (H3C)2CuLi an enolatearrow_forward
- b. H3C CH3 1. 2. H3O+ H3C MgBr H3Carrow_forwardPredict the major products of this reaction: excess H+ NaOH ? A Note that the first reactant is used in excess, that is, there is much more of the first reactant than the second. If there won't be any products, just check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privarrow_forward1. For each of the reaction "railroads" below, you are either asked to give the structure(s) of the starting material(s) or product(s), or provide reagents/conditions to accomplish the transformation, as indicated by the boxes. a. NaOMe H+ .CO,H HO₂C MeOH (excess) MeOH H3C Br يع CH3 1. LiAlH4 2. H3O+ 3. PBг3 H3C 1. Et-Li 2. H3O+ -CO₂Me -CO₂Me OH CH3 CH3 ল CH3arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
