
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 2PP
PRACTICE PROBLEM
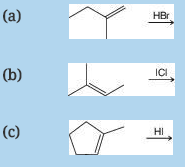
Outline mechanisms for the following addition reactions:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please help me figure out the mechanism with arrows of the following reaction
Organic Functional Groups
Predicting the reactants or products of acetal hydrolysis
termine the structures of the missing organic molecules in the following reaction:
H*
H*
+ H₂O
Y
☑
Note: Molecules that share the same letter have the exact same structure.
In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X, Y, and Z. You may draw
that you like, so long as they aren't touching. Molecule X shows up in multiple steps, but you only have to draw its structure
Explanation
Check
@2
W
Click and drag to start drawing a structure.
#4
# 3
LU
E
%
67 olo
5
66
R
T
Y
&
7
AcGraw Hill LLC. All Rights R
X
8. (16 pts) Provide the stepwise mechanism for the synthesis of the following compound via an enamine
Chapter 8 Solutions
Organic Chemistry
Ch. 8 - Prob. 1PPCh. 8 - PRACTICE PROBLEM Outline mechanisms for the...Ch. 8 - Practice Problem 8.3 Provide mechanistic...Ch. 8 - Prob. 4PPCh. 8 - PRACTICE PROBLEM
8.5 In one industrial synthesis...Ch. 8 - Prob. 6PPCh. 8 - Prob. 7PPCh. 8 - Prob. 8PPCh. 8 - Prob. 9PPCh. 8 - PRACTICE PROBLEM (a) Outline a likely mechanism...
Ch. 8 - Prob. 11PPCh. 8 - Prob. 12PPCh. 8 - Practice Problem 8.13
Specify the appropriate...Ch. 8 - Prob. 14PPCh. 8 - Practice Problem 8.15 Write a mechanism to explain...Ch. 8 - Prob. 16PPCh. 8 - Prob. 17PPCh. 8 - Prob. 18PPCh. 8 - Practice Problem 8.19 Treating cyclohexene with l,...Ch. 8 - Prob. 20PPCh. 8 - Practice Problem 8.21
Predict the products of the...Ch. 8 - Prob. 22PPCh. 8 - Prob. 23PPCh. 8 - Prob. 24PPCh. 8 - Prob. 25PPCh. 8 - Write structural formulas for the products that...Ch. 8 - Prob. 27PCh. 8 - Prob. 28PCh. 8 - 8.29. Give the structure of the products that you...Ch. 8 - Give the structure of the products you would...Ch. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - Prob. 34PCh. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - When 3, 3-dimethyl-2-butanol is neared with...Ch. 8 - Prob. 39PCh. 8 - Prob. 40PCh. 8 - Prob. 41PCh. 8 - The reaction of bromine with cyclohexene involves...Ch. 8 - Prob. 43PCh. 8 - Internal alkynes can be isomerized to terminal...Ch. 8 - 8.43. Write a mechanism that explains the...Ch. 8 - 8.44. Write a mechanism for the following...Ch. 8 - Write a mechanism that explains formation of the...Ch. 8 - Prob. 48PCh. 8 - 8.49 Farnesene (below) is a compound found in the...Ch. 8 - Prob. 50PCh. 8 - Limonene is a compound found in orange oil and...Ch. 8 - Prob. 52PCh. 8 - Synthesize the following compound starting with...Ch. 8 - Prob. 54PCh. 8 - Predict features of their IR spectra that you...Ch. 8 - Deduce the structures of compounds A, B, and C,...Ch. 8 - Ricinoleic acid, a compound that can be isolated...Ch. 8 - 8.54. There are two dicarboxylic acids with the...Ch. 8 - Prob. 59PCh. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 64PCh. 8 - Prob. 66PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - 8.65
(a)Based on the following information, draw...Ch. 8 - Triethylamine, (C2H5)3N, like all amines, has a...Ch. 8 - (a) Synthesize (3S, 4R)-3,...Ch. 8 - Prob. 2LGPCh. 8 - Prob. 3LGPCh. 8 - Prob. 4LGPCh. 8 - 8.1 A hydrocarbon whose molecular formula is...Ch. 8 - Prob. 2QCh. 8 - Give the major product of the reaction of...Ch. 8 - The compound shown here is best prepared by which...Ch. 8 - 8.5 A compound whose formula is C6H10 (Compound A)...Ch. 8 - Prob. 6QCh. 8 - 8.7 Which reaction sequence converts cyclohexene...Ch. 8 - Which of the following sequences leads to the best...
Additional Science Textbook Solutions
Find more solutions based on key concepts
At what time does the warmest temperature occur?
Applications and Investigations in Earth Science (9th Edition)
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Community 1 contains 100 individuals distributed among four species: 5A, 5B, 85C, and 5D Community 2 contains 1...
Campbell Biology in Focus (2nd Edition)
The bioremediation process shown in the photograph is used to remove benzene and other hydrocarbons from soil c...
Microbiology: An Introduction
Researchers cross a corn plant that is pure - breeding forthe dominant traits colored aleurone (C1), full kerne...
Genetic Analysis: An Integrated Approach (3rd Edition)
In what ways does connective tissue differ from epithelial tissue?
Principles of Anatomy and Physiology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the titration curve of (i) weak acid vs. strong base; (ii) weak acid vs. weakbase; (iii) diprotic acid with strong base (iii) triprotic acid with strong base.arrow_forwardComplete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. More... No reaction. my ㄖˋ + 1. Na O Me Click and drag to start drawing a structure. 2. H +arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe H+ + 1 2 H H work up You can draw 1 and 2 in any arrangement you like. Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Click and drag to start drawing a structure. X $ dmarrow_forward
- Predict the major products of this organic reaction: 1. NaH (20°C) 2. CH3Br ? Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. G Crarrow_forwardPredict the major products of this organic reaction: 1. LDA (-78°C) ? 2. Br Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. . • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. Xarrow_forwardPlease draw the structuresarrow_forward
- Draw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 0 1. Eto 1. Eto- 1 2 2. MeBr 2. EtBr H3O+ A 3 You can draw the three structures in any arrangement you like. Explanation Check Click and drag to start drawing a structure.arrow_forwardDraw the missing intermediate 1 and final product 2 of this synthesis: 1. MeO- H3O+ 1 2 2. PrBr Δ You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardWhat is the differences between: Glyceride and phosphoglyceride Wax and Fat Soap and Fatty acid HDL and LDL cholesterol Phospho lipids and sphingosine What are the types of lipids? What are the main lipid components of membrane structures? How could lipids play important rules as signaling molecules and building units? The structure variety of lipids makes them to play significant rules in our body, conclude breifly on this statement.arrow_forward
- What is the differences between DNA and RNA for the following: - structure - function - type What is the meaning of: - replication - transcription - translation show the base pair connection(hydrogen bond) in DNA and RNAarrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License