
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 3PP
Practice Problem 8.3
Provide mechanistic explanations for the following observations:
(a)
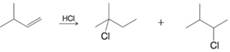
(b)
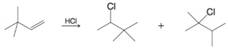
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
esc
2
Incorrect
Feedback: Your answer is incorrect.
Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating
the reactants?
? A
O
• If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like.
. If your answer is no, check the box under the drawing area instead.
Check
F1
!
@
X
C
Save For Later
Submit Assignment
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
80
et
A
ད
1
4
F2
F3
F4
F5
F6
F7
F8
F9
F10
F11
F12
#
$
45
%
A
6
87
&
*
8
9
)
0
+ ||
Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating
the reactants?
?A
Δ
O
• If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like.
• If your answer is no, check the box under the drawing area instead.
Explanation
Check
Click and drag to start drawing a structure.
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilit
ku
F11
१
eq
ine teaching and × +
rn/takeAssignment/takeCovalentActivity.do?locator-assignment-take
[Review Topics]
[References]
Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.)
Keep the information page open for feedback reference.
The IUPAC name is
In progress
mit Answer
Retry Entire Group
5 more group attempts remaining
Cengage Learning | Cengage Technical Support
Save and Exit
Chapter 8 Solutions
Organic Chemistry
Ch. 8 - Prob. 1PPCh. 8 - Prob. 2PPCh. 8 - Practice Problem 8.3 Provide mechanistic...Ch. 8 - Prob. 4PPCh. 8 - Prob. 5PPCh. 8 - Prob. 6PPCh. 8 - Prob. 7PPCh. 8 - Prob. 8PPCh. 8 - Prob. 9PPCh. 8 - Prob. 10PP
Ch. 8 - Prob. 11PPCh. 8 - Prob. 12PPCh. 8 - Practice Problem 8.13
Specify the appropriate...Ch. 8 - Prob. 14PPCh. 8 - Practice Problem 8.15 Write a mechanism to explain...Ch. 8 - Prob. 16PPCh. 8 - Prob. 17PPCh. 8 - Prob. 18PPCh. 8 - Practice Problem 8.19 Treating cyclohexene with l,...Ch. 8 - Prob. 20PPCh. 8 - Practice Problem 8.21
Predict the products of the...Ch. 8 - Prob. 22PPCh. 8 - Prob. 23PPCh. 8 - Prob. 24PPCh. 8 - Prob. 25PPCh. 8 - Write structural formulas for the products that...Ch. 8 - Prob. 27PCh. 8 - Prob. 28PCh. 8 - 8.29. Give the structure of the products that you...Ch. 8 - Give the structure of the products you would...Ch. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - Prob. 34PCh. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - When 3, 3-dimethyl-2-butanol is neared with...Ch. 8 - Prob. 39PCh. 8 - Prob. 40PCh. 8 - Prob. 41PCh. 8 - Prob. 42PCh. 8 - 8.43. Write a mechanism that explains the...Ch. 8 - 8.44. Write a mechanism for the following...Ch. 8 - Write a mechanism that explains formation of the...Ch. 8 - Prob. 46PCh. 8 - 8.47. Farnesene (below) is a compound found in the...Ch. 8 - Limonene is a compound found in orange oil and...Ch. 8 - Prob. 49PCh. 8 - Synthesize the following compound starting with...Ch. 8 - Predict features of their IR spectra that you...Ch. 8 - Deduce the structures of compounds A, B, and C,...Ch. 8 - Ricinoleic acid, a compound that can be isolated...Ch. 8 - 8.54. There are two dicarboxylic acids with the...Ch. 8 - Prob. 55PCh. 8 - Prob. 56PCh. 8 - Prob. 57PCh. 8 - Prob. 58PCh. 8 - 8.59. For each of the following questions, please...Ch. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Triethylamine, (C2H5)3N, like all amines, has a...Ch. 8 - (a) Synthesize (3S, 4R)-3,...Ch. 8 - Prob. 2LGPCh. 8 - Prob. 3LGPCh. 8 - Prob. 4LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
GO In Fig. 8-55, a block slides along a path that is without friction until the block reaches the section of le...
Fundamentals of Physics Extended
All of the following processes are involved in the carbon cycle except: a. photosynthesis b. cell respiration c...
Human Biology: Concepts and Current Issues (8th Edition)
18. SCIENTIFIC THINKING By measuring the fossil remains of Homo floresiensis, scientists have estimated its wei...
Campbell Biology: Concepts & Connections (9th Edition)
13. The displacement of a wave traveling in thee positive x-direction is D(x, t) = (3.5cm) sin(17x – 124t), whe...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Whether carbon, C, is more similar to nitrogen, N, O, or silicon, Si is to be explained. Concept introduction: ...
Living By Chemistry: First Edition Textbook
Flask A contains yeast cells in glucose-minimal salts broth incubated at 30C with aeration. Flask B contains ye...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the molecules.arrow_forwardDraw the mechanism for the acid-catalyzed dehydration of 2-methyl-hexan-2-ol with arrows please.arrow_forward. Draw the products for addition reactions (label as major or minor) of the reaction between 2-methyl-2-butene and with following reactants : Steps to follow : A. These are addition reactions you need to break a double bond and make two products if possible. B. As of Markovnikov rule the hydrogen should go to that double bond carbon which has more hydrogen to make stable products or major product. Here is the link for additional help : https://study.com/academy/answer/predict-the-major-and-minor-products-of-2-methyl- 2-butene-with-hbr-as-an-electrophilic-addition-reaction-include-the-intermediate- reactions.html H₂C CH3 H H3C CH3 2-methyl-2-butene CH3 Same structure CH3 IENCESarrow_forward
- Draw everything on a piece of paper including every single step and each name provided using carbons less than 3 please.arrow_forwardTopics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. H The IUPAC name isarrow_forward[Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forward
- Please draw.arrow_forwardA chromatogram with ideal Gaussian bands has tR = 9.0 minutes and w1/2 = 2.0 minutes. Find the number of theoretical plates that are present, and calculate the height of each theoretical plate if the column is 10 centimeters long.arrow_forwardAn open tubular column has an inner diameter of 207 micrometers, and the thickness of the stationary phase on the inner wall is 0.50 micrometers. Unretained solute passes through in 63 seconds and a particular solute emerges at 433 seconds. Find the distribution constant for this solute and find the fraction of time spent in the stationary phase.arrow_forward
- Consider a chromatography column in which Vs= Vm/5. Find the retention factor if Kd= 3 and Kd= 30.arrow_forwardTo improve chromatographic separation, you must: Increase the number of theoretical plates on the column. Increase the height of theoretical plates on the column. Increase both the number and height of theoretical plates on the column. Increasing the flow rate of the mobile phase would Increase longitudinal diffusion Increase broadening due to mass transfer Increase broadening due to multiple paths You can improve the separation of components in gas chromatography by: Rasing the temperature of the injection port Rasing the temperature of the column isothermally Rasing the temperature of the column using temperature programming In GC, separation between two different solutes occurs because the solutes have different solubilities in the mobile phase the solutes volatilize at different rates in the injector the solutes spend different amounts of time in the stationary phasearrow_forwardplease draw and example of the following: Show the base pair connection(hydrogen bond) in DNA and RNAarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY