
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 15PP
Practice Problem 8.15
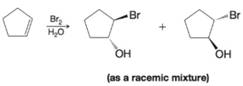
Write a mechanism to explain the following reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Seee the attached ima
Please see the attached image.
Please see the attached image.
Chapter 8 Solutions
Organic Chemistry
Ch. 8 - Prob. 1PPCh. 8 - Prob. 2PPCh. 8 - Practice Problem 8.3 Provide mechanistic...Ch. 8 - Prob. 4PPCh. 8 - Prob. 5PPCh. 8 - Prob. 6PPCh. 8 - Prob. 7PPCh. 8 - Prob. 8PPCh. 8 - Prob. 9PPCh. 8 - Prob. 10PP
Ch. 8 - Prob. 11PPCh. 8 - Prob. 12PPCh. 8 - Practice Problem 8.13
Specify the appropriate...Ch. 8 - Prob. 14PPCh. 8 - Practice Problem 8.15 Write a mechanism to explain...Ch. 8 - Prob. 16PPCh. 8 - Prob. 17PPCh. 8 - Prob. 18PPCh. 8 - Practice Problem 8.19 Treating cyclohexene with l,...Ch. 8 - Prob. 20PPCh. 8 - Practice Problem 8.21
Predict the products of the...Ch. 8 - Prob. 22PPCh. 8 - Prob. 23PPCh. 8 - Prob. 24PPCh. 8 - Prob. 25PPCh. 8 - Write structural formulas for the products that...Ch. 8 - Prob. 27PCh. 8 - Prob. 28PCh. 8 - 8.29. Give the structure of the products that you...Ch. 8 - Give the structure of the products you would...Ch. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - Prob. 34PCh. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - When 3, 3-dimethyl-2-butanol is neared with...Ch. 8 - Prob. 39PCh. 8 - Prob. 40PCh. 8 - Prob. 41PCh. 8 - Prob. 42PCh. 8 - 8.43. Write a mechanism that explains the...Ch. 8 - 8.44. Write a mechanism for the following...Ch. 8 - Write a mechanism that explains formation of the...Ch. 8 - Prob. 46PCh. 8 - 8.47. Farnesene (below) is a compound found in the...Ch. 8 - Limonene is a compound found in orange oil and...Ch. 8 - Prob. 49PCh. 8 - Synthesize the following compound starting with...Ch. 8 - Predict features of their IR spectra that you...Ch. 8 - Deduce the structures of compounds A, B, and C,...Ch. 8 - Ricinoleic acid, a compound that can be isolated...Ch. 8 - 8.54. There are two dicarboxylic acids with the...Ch. 8 - Prob. 55PCh. 8 - Prob. 56PCh. 8 - Prob. 57PCh. 8 - Prob. 58PCh. 8 - 8.59. For each of the following questions, please...Ch. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Triethylamine, (C2H5)3N, like all amines, has a...Ch. 8 - (a) Synthesize (3S, 4R)-3,...Ch. 8 - Prob. 2LGPCh. 8 - Prob. 3LGPCh. 8 - Prob. 4LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Assume we have three states of saturated vapor R-134a at +40C,0C, and 40C . Calculate the specific volume at th...
Fundamentals Of Thermodynamics
For parts a, b, and c, draw a diagram illustrating the alleleson homologous chromosomes for the following genot...
Genetic Analysis: An Integrated Approach (3rd Edition)
Why are the top predators in food chains most severely affected by pesticides such as DDT?
Campbell Essential Biology (7th Edition)
Microphylls are found in which plant group? (A) lycophytes (B) liverworts (C) ferns (D) hornworts
Campbell Biology (11th Edition)
8. A human maintaining a vegan diet (containing no animal products) would be a:
a. producer
b. primary consume...
Human Biology: Concepts and Current Issues (8th Edition)
1. Which is a function of the skeletal system? (a) support, (b) hematopoietic site, (c) storage, (d) providing ...
Anatomy & Physiology (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- V Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forwardComplete the mechanismarrow_forwardComplete the mechanismarrow_forward
- 8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forwardJust try completing it and it should be straightforward according to the professor and TAs.arrow_forwardThe grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY