
Concept explainers
Draw structures corresponding to the following systematic names:
(a) (4E)-2, 4-Dimethyl-l, 4-hexadiene
(b) cis-3, 3-Dimethyl-4-propyl-l, 5-octadiene
(c) 4-Methyl-l, 2-pentadiene
(d) (3E, 5Z)-2, 6-Dimethyl-1, 3, 5, 7-octatetraene
(e) 3-Butyl-2-heptene
(f) trans-2, 2, 5, 5-Tetramethyl-3-hexene
a) (4E)-2, 4-Dimethyl-1, 4-hexadiene.
Interpretation:
The structure corresponding to the systematic name (4E)-2, 4-dimethyl-1, 4-hexadiene is to be drawn.
Concept introduction:
The longest carbon chain containing the double bond to be chosen. Based on the name of the parent compound–the alkene name ends with the suffix–alkene. The chain is to be numbered from the end that gives the lowest number to the carbon in double bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the double bond is indicated by giving the number of the first alkene carbon before the name of the parent name. If more than one double bond is present, their positions are indicated with the suffixes -diene, -triene and so on. The isomer that has the higher ranked groups on each carbon are on the same side of the double bond is said to have Z configuration. If the higher ranked groups are on opposite sides, the alkene is said to have E configuration.
To draw:
The structure corresponding to the systematic name (4E)-2, 4-dimethyl-1, 4-hexadiene.
Answer to Problem 38AP
The structure corresponding to the systematic name (4E)-2, 4-dimethyl-1, 4-hexadiene is
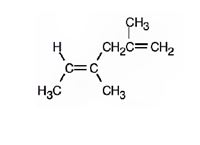
Explanation of Solution
The name shows that the longest carbon chain should contain six carbons with two double bonds between C2 & C3 and C4 & C5. There should be two methyl substituents one on C2 and other on C4. Further higher ranked groups on each carbon should be in the opposite sides of the double bonds.
The structure corresponding to the systematic name (4E)-2, 4-dimethyl-1, 4-hexadiene is

b) cis-3, 3-Dimethyl-4-isopropyl-1, 5-octadiene.
Interpretation:
The structure corresponding to the systematic name cis-3, 3-dimethyl-4-isopropyl-1, 5-octadiene is to be drawn.
Concept introduction:
The longest carbon chain containing the double bond to be chosen. Based on the name of the parent compound–the alkene name ends with the suffix–ene. The chain is to be numbered from the end that gives the lowest number to the carbon in double bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the double bond is indicated by giving the number of the first alkene carbon before the name of the parent name. If more than one double bond is present, their positions are indicated with the suffixes -diene, -triene and so on. The isomer that has similar groups on each carbon on the same side of the double bond is called as the cis isomer. The isomer that has similar groups on each carbon on the opposite side of the double bond is called as thetrans isomer.
To draw:
The structure corresponding to the systematic name cis-3, 3-dimethyl-4-isopropyl-1, 5-octadiene.
Answer to Problem 38AP
The structure corresponding to the systematic name cis-3, 3-dimethyl-4-isopropyl-1, 5-octadiene is
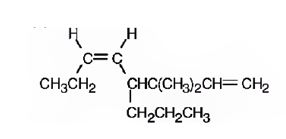
Explanation of Solution
The name shows that the longest carbon chain should contain eight carbons with two double bonds between C1 & C2 and C5 & C6. There should be two methyl substituents on C3 and an isopropyl group on C4. Further similar groups in each carbon should be on the same sides of the double bonds.
The structure corresponding to the systematic name cis-3, 3-dimethyl-4-isopropyl-1, 5-octadiene is

c) 4-Methyl-1, 2-pentadiene.
Interpretation:
The structure corresponding to the systematic name4-methyl-1, 2-pentadiene is to be drawn.
Concept introduction:
The longest carbon chain containing the double bond to be chosen. Based on the name of the parent compound–the alkene name ends with the suffix–ene. The chain is to be numbered from the end that gives the lowest number to the carbon in double bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the double bond is indicated by giving the number of the first alkene carbon before the name of the parent name. If more than one double bond is present, their positions are indicated with the suffixes -diene, -triene and so on.
To draw:
The structure corresponding to the systematic name 4-methyl-1, 2-pentadiene.
Answer to Problem 38AP
The structure corresponding to the systematic name 4-methyl-1, 2-pentadiene is
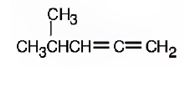
Explanation of Solution
The name shows that the longest carbon chain should contain five carbons with two double bonds between C1 & C2 and C2 & C3. There should be amethyl substituent on C4.
The structure corresponding to the systematic name 4-methyl-1, 2-pentadiene is
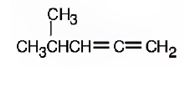
d) (3E, 5Z)-2, 6-Dimethyl-1, 3, 5, 7-octatetraene.
Interpretation:
The structure corresponding to the systematic name (3E, 5Z)-2, 6-dimethyl-1, 3, 5, 7-octatetraene is to be drawn.
Concept introduction:
The longest carbon chain containing the double bond to be chosen. Based on the name of the parent compound–the alkene name ends with the suffix–ene. The chain is to be numbered from the end that gives the lowest number to the carbon in double bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the double bond is indicated by giving the number of the first alkene carbon before the name of the parent name. If more than one double bond is present, their positions are indicated with the suffixes -diene, -triene and so on. The isomer that has the higher ranked groups on each carbon are on the same side of the double bond is said to have Z configuration. If the higher ranked groups are on opposite sides, the alkene is said to have E configuration.
To draw:
The structure corresponding to the systematic name (3E, 5Z)-2, 6-dimethyl-1, 3, 5, 7-octatetraene.
Answer to Problem 38AP
The structure corresponding to the systematic name (3E, 5Z)-2, 6-dimethyl-1, 3, 5, 7-octatetraene is
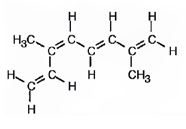
Explanation of Solution
The name shows that the longest carbon chain should contain eight carbons with two double bonds between C2 & C3 and C6 & C7. There should be two methyl substituents one on C2 and other on C6. Further higher ranked groups on C2 & C3 must be placed on the opposite sides of the double bond. The higher ranked groups on C6 & C7 must be placed on the same side of the double bond.
The structure corresponding to the systematic name (3E, 5Z)-2, 6-dimethyl-1, 3, 5, 7-octatetraene is

e) 3-Butyl-2-heptene.
Interpretation:
The structure corresponding to the systematic name3-butyl-2-heptene is to be drawn.
Concept introduction:
The longest carbon chain containing the double bond to be chosen. Based on the name of the parent compound–the alkene name ends with the suffix–ene. The chain is to be numbered from the end that gives the lowest number to the carbon in double bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the double bond is indicated by giving the number of the first alkene carbon before the name of the parent name. If more than one double bond is present, their positions are indicated with the suffixes -diene, -triene and so on.
To draw:
The structure corresponding to the systematic name 3-butyl-2-heptene.
Answer to Problem 38AP
The structure corresponding to the systematic name 3-butyl-2-heptene is
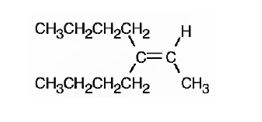
Explanation of Solution
The name shows that the longest carbon chain should contain seven carbons with a double bond between C2 & C3. There should be a butyl substituent on C3.
The structure corresponding to the systematic name 3-butyl-2-heptene is

f) trans-2, 2, 5, 5-Tetramethyl-3-hexene.
Interpretation:
The structure corresponding to the systematic name trans-2, 2, 5, 5-tetramethyl-3-hexene is to be drawn.
Concept introduction:
The longest carbon chain containing the double bond to be chosen. Based on the name of the parent compound–the alkene name ends with the suffix–ene. The chain is to be numbered from the end that gives the lowest number to the carbon in double bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the double bond is indicated by giving the number of the first alkene carbon before the name of the parent name. If more than one double bond is present, their positions are indicated with the suffixes -diene, -triene and so on. The isomer that has similar groups on each carbon on the same side of the double bond is called as the cis isomer. The isomer that has similar groups on each carbon on the opposite side of the double bond is called as the trans isomer.
To draw:
The structure corresponding to the systematic name trans-2, 2, 5, 5-tetramethyl-3-hexene.
Answer to Problem 38AP
The structure corresponding to the systematic name trans-2, 2, 5, 5-tetramethyl-3-hexene is
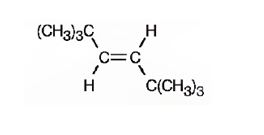
Explanation of Solution
The name shows that the longest carbon chain should contain six carbons with a double bond between C3 & C4. There should be four methyl substituents two on C3 and two on C5. Further similar groups in each carbon should be on the opposite sides of the double bonds.
The structure corresponding to the systematic name trans-2,2,5,5-tetramethyl-3-hexene is

Want to see more full solutions like this?
Chapter 7 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- Can you please help me with this problem and explain it step by step? I'm so confused about itarrow_forward2. Identify the reagents you would need to achieve the following. You may need to consider using a protecting group. HO 1. 2. 3. 4. 5. OH Br HOarrow_forwardBeF2 exists as a linear molecule. Which kind of hybrid orbitals does Be use in this compound? Use Orbital Diagrams to show how the orbitals are formed. (6)arrow_forward
- Please answer the questions and provide detailed explanations as well as a drawing to show the signals in the molecule.arrow_forwardPropose an efficient synthesis for the following transformation: EN The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. t-BuOK B. Na2Cr2O7, H2SO4, H2O C. NBS, heat F. NaCN D. MeOH E. NaOH G. MeONa H. H2O I. 1) O3; 2) DMSarrow_forwardStereochemistry Identifying the enantiomer of a simple organic molecule 1/5 Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of t above box under the table. Br ま HO H 0 Molecule 1 Molecule 2 Molecule 3 OH H Br H H" Br OH Br Molecule 4 Br H OH + + OH Molecule 5 Br H OH none of the above Molecule 6 Br H... OHarrow_forward
- Please answer the questions and provide detailed explanations.arrow_forwardQuestion 16 0/1 pts Choose the correct option for the following cycloaddition reaction. C CF3 CF3 CF3 CF3 The reaction is suprafacial/surafacial and forbidden The reaction is antarafacial/antarafacial and forbidden The reaction is antarafacial/antarafacial and allowed The reaction is suprafacial/surafacial and allowedarrow_forward1. Give the structures of the products obtained when the following are heated. Include stereochemistry where relevant. A NO2 + NO2 B + C N=C CEN + { 2. Which compounds would you heat together in order to synthesize the following?arrow_forward
- Explain how myo-inositol is different from D-chiro-inositol. use scholarly sources and please hyperlink.arrow_forwardWhat is the molarisuty of a 0.396 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 /mol.arrow_forwardProvide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly. Br ......Im OHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





