
General, Organic, and Biological Chemistry
7th Edition
ISBN: 9781285853918
Author: H. Stephen Stoker
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 7.9, Problem 2QQ
Interpretation Introduction
Interpretation:
Among the given options, which one belongs to changes of state is the final state that of solid has to be chosen.
Concept Introduction:
A change of state is a cycle in that a substance is transformed from one physical state to another physical sate. The physical changes of a substance occurs by either heating or cooling. There are six possible changes of state and they are freezing, melting, evaporation, condensation, sublimation, and deposition. The below mentioned figure will represent each of the physical changes of a substance from starting stage to final stage via intermediate stage.
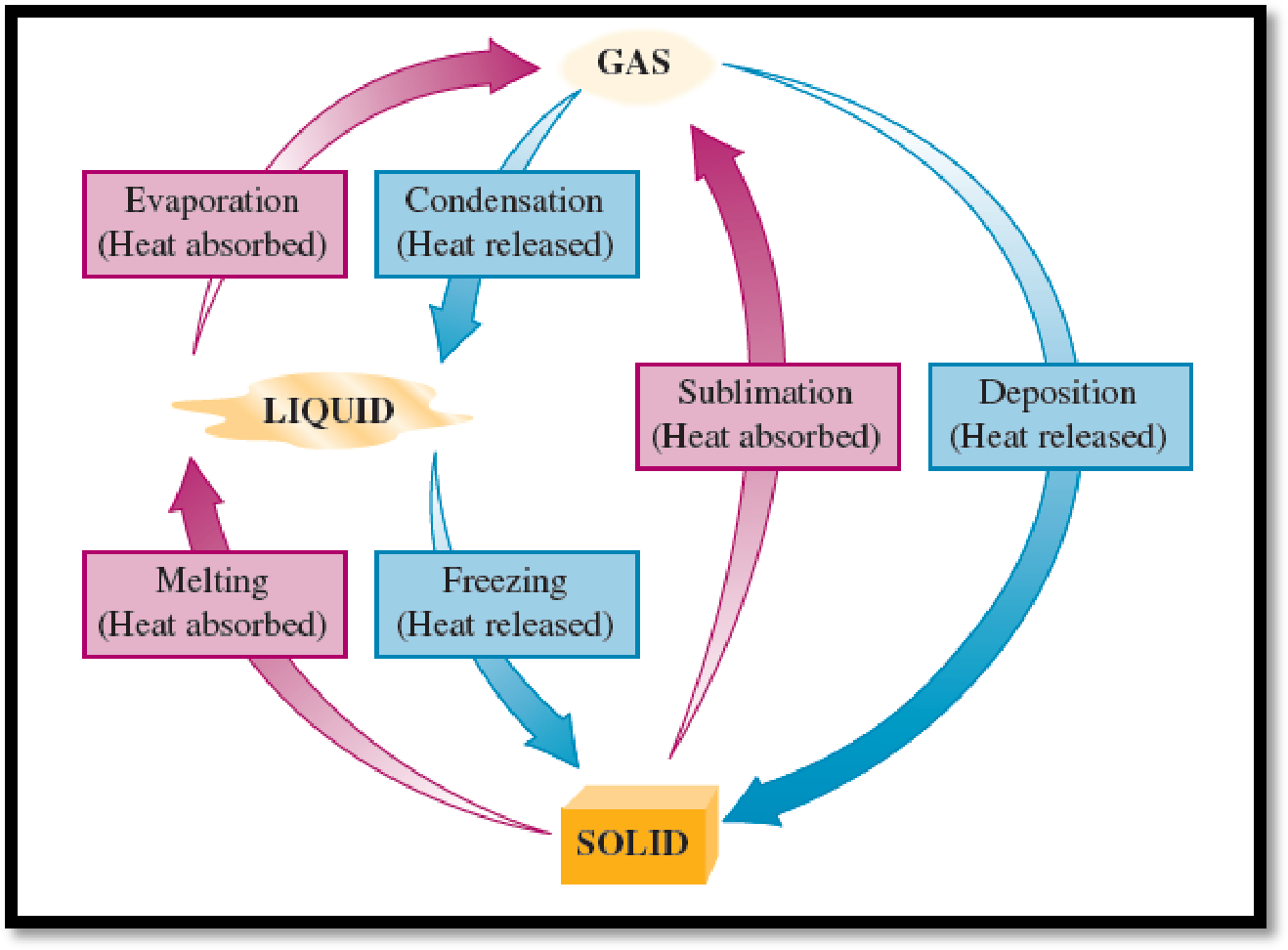
Figure 1
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the Nernst equation to calculate nonstandard cell voltage
A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction:
MnO2 (s)+4H* (aq)+2Cr²+ (aq) → Mn²+ (aq)+2H₂O (1)+2Cr³+ (aq)
+
2+
2+
3+
Suppose the cell is prepared with 7.44 M H* and 0.485 M Cr²+ in one half-cell and 7.92 M Mn² and 3.73 M Cr³+ in the other.
Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.
☐
x10
μ
Х
5
?
000
日。
Calculating standard reaction free energy from standard reduction...
Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction.
Be sure your answer has the correct number of significant digits.
NO (g) +H₂O (1) + Cu²+ (aq) → HNO₂ (aq) +H* (aq)+Cu* (aq)
kJ
-
☐ x10
x10
olo
18
Ar
Calculating the pH of a weak base titrated with a strong acid
b
An analytical chemist is titrating 116.9 mL of a 0.7700M solution of aniline (C6H5NH2) with a 0.5300M solution of HNO3. The pK of aniline is 9.37.
Calculate the pH of the base solution after the chemist has added 184.2 mL of the HNO 3 solution to it.
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added.
Round your answer to 2 decimal places.
pH = ☐
☑
5
Chapter 7 Solutions
General, Organic, and Biological Chemistry
Ch. 7.1 - Prob. 1QQCh. 7.1 - Prob. 2QQCh. 7.1 - Prob. 3QQCh. 7.2 - Prob. 1QQCh. 7.2 - Prob. 2QQCh. 7.2 - Prob. 3QQCh. 7.3 - Prob. 1QQCh. 7.3 - Prob. 2QQCh. 7.3 - Prob. 3QQCh. 7.4 - Prob. 1QQ
Ch. 7.4 - Prob. 2QQCh. 7.4 - Based on Boyles law, if the pressure on 30.0 mL of...Ch. 7.5 - Prob. 1QQCh. 7.5 - Prob. 2QQCh. 7.5 - Prob. 3QQCh. 7.6 - Prob. 1QQCh. 7.6 - Prob. 2QQCh. 7.6 - Prob. 3QQCh. 7.7 - Prob. 1QQCh. 7.7 - Prob. 2QQCh. 7.7 - Prob. 3QQCh. 7.7 - Prob. 4QQCh. 7.8 - Prob. 1QQCh. 7.8 - Prob. 2QQCh. 7.8 - Prob. 3QQCh. 7.9 - Prob. 1QQCh. 7.9 - Prob. 2QQCh. 7.9 - Prob. 3QQCh. 7.10 - Prob. 1QQCh. 7.10 - Prob. 2QQCh. 7.10 - Prob. 3QQCh. 7.11 - Prob. 1QQCh. 7.11 - Prob. 2QQCh. 7.11 - Prob. 3QQCh. 7.11 - Prob. 4QQCh. 7.11 - Prob. 5QQCh. 7.11 - Prob. 6QQCh. 7.12 - Prob. 1QQCh. 7.12 - Prob. 2QQCh. 7.12 - Prob. 3QQCh. 7.13 - Prob. 1QQCh. 7.13 - Prob. 2QQCh. 7.13 - Prob. 3QQCh. 7.13 - Prob. 4QQCh. 7.13 - Prob. 5QQCh. 7.13 - Prob. 6QQCh. 7 - Indicate whether each of the following statements...Ch. 7 - Indicate whether each of the following statements...Ch. 7 - Prob. 7.3EPCh. 7 - Prob. 7.4EPCh. 7 - Prob. 7.5EPCh. 7 - Prob. 7.6EPCh. 7 - Prob. 7.7EPCh. 7 - Prob. 7.8EPCh. 7 - Prob. 7.9EPCh. 7 - Prob. 7.10EPCh. 7 - Prob. 7.11EPCh. 7 - Prob. 7.12EPCh. 7 - Prob. 7.13EPCh. 7 - Prob. 7.14EPCh. 7 - Prob. 7.15EPCh. 7 - Prob. 7.16EPCh. 7 - Prob. 7.17EPCh. 7 - Prob. 7.18EPCh. 7 - A sample of ammonia (NH3), a colorless gas with a...Ch. 7 - A sample of nitrogen dioxide (NO2), a toxic gas...Ch. 7 - Prob. 7.21EPCh. 7 - Prob. 7.22EPCh. 7 - Prob. 7.23EPCh. 7 - Prob. 7.24EPCh. 7 - Prob. 7.25EPCh. 7 - Prob. 7.26EPCh. 7 - A sample of N2 gas occupies a volume of 375 mL at...Ch. 7 - A sample of Ar gas occupies a volume of 1.2 L at...Ch. 7 - Prob. 7.29EPCh. 7 - Prob. 7.30EPCh. 7 - Prob. 7.31EPCh. 7 - Prob. 7.32EPCh. 7 - Prob. 7.33EPCh. 7 - Prob. 7.34EPCh. 7 - Prob. 7.35EPCh. 7 - Prob. 7.36EPCh. 7 - Prob. 7.37EPCh. 7 - Prob. 7.38EPCh. 7 - Prob. 7.39EPCh. 7 - Prob. 7.40EPCh. 7 - Prob. 7.41EPCh. 7 - Prob. 7.42EPCh. 7 - Prob. 7.43EPCh. 7 - Prob. 7.44EPCh. 7 - Prob. 7.45EPCh. 7 - Prob. 7.46EPCh. 7 - Prob. 7.47EPCh. 7 - Prob. 7.48EPCh. 7 - Prob. 7.49EPCh. 7 - Prob. 7.50EPCh. 7 - Determine the following for a 0.250-mole sample of...Ch. 7 - Determine the following for a 0.500-mole sample of...Ch. 7 - Prob. 7.53EPCh. 7 - Prob. 7.54EPCh. 7 - Prob. 7.55EPCh. 7 - What is the value of the ideal gas constant R if...Ch. 7 - The total pressure exerted by a mixture of O2, N2,...Ch. 7 - The total pressure exerted by a mixture of He, Ne,...Ch. 7 - A gas mixture contains O2, N2, and Ar at partial...Ch. 7 - A gas mixture contains He, Ne, and H2S at partial...Ch. 7 - Prob. 7.61EPCh. 7 - Prob. 7.62EPCh. 7 - Prob. 7.63EPCh. 7 - Prob. 7.64EPCh. 7 - Prob. 7.65EPCh. 7 - Prob. 7.66EPCh. 7 - Prob. 7.67EPCh. 7 - Prob. 7.68EPCh. 7 - Prob. 7.69EPCh. 7 - Prob. 7.70EPCh. 7 - Prob. 7.71EPCh. 7 - Prob. 7.72EPCh. 7 - What are the two ways in which the escape of...Ch. 7 - Prob. 7.74EPCh. 7 - Prob. 7.75EPCh. 7 - How does an increase in the surface area of a...Ch. 7 - Prob. 7.77EPCh. 7 - Prob. 7.78EPCh. 7 - Prob. 7.79EPCh. 7 - Prob. 7.80EPCh. 7 - Prob. 7.81EPCh. 7 - What is the relationship between the strength of...Ch. 7 - What term is used to describe a substance that...Ch. 7 - Prob. 7.84EPCh. 7 - Indicate whether each of the following statements...Ch. 7 - Indicate whether each of the following statements...Ch. 7 - Prob. 7.87EPCh. 7 - What is the relationship between location...Ch. 7 - Prob. 7.89EPCh. 7 - Prob. 7.90EPCh. 7 - Indicate whether or not each of the following...Ch. 7 - Prob. 7.92EPCh. 7 - Prob. 7.93EPCh. 7 - Prob. 7.94EPCh. 7 - For liquid-state samples of the following diatomic...Ch. 7 - For liquid-state samples of the following diatomic...Ch. 7 - Prob. 7.97EPCh. 7 - Prob. 7.98EPCh. 7 - Prob. 7.99EPCh. 7 - Prob. 7.100EPCh. 7 - Prob. 7.101EPCh. 7 - Prob. 7.102EPCh. 7 - Prob. 7.103EPCh. 7 - Prob. 7.104EPCh. 7 - Prob. 7.105EPCh. 7 - Prob. 7.106EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- QUESTION: Find the standard deviation for the 4 different groups 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5.033 4.044 334.6 268.7 4.706 3.621 305.6 234.4 4.816 3.728 340.0 262.7 4.828 4.496 304.3 283.2 4.993 3.865 244.7 143.6 STDEV = STDEV = STDEV = STDEV =arrow_forwardQUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression' *The images of the data showing 'coefficients for the standard curve' have been providedarrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage Try Again Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ 2+ Sn²+ Ba(s) (aq) + Ba (s) Sn (s) + Ba²+ (aq) →>> Suppose the cell is prepared with 6.10 M Sn 2+ 2+ in one half-cell and 6.62 M Ba in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 1.71 V ☐ x10 ☑ 5 0/5 ? 00. 18 Ararrow_forward
- Question: Find both the b (gradient) and a (y-intercept) value from the list of data below: (x1 -x̄) 370.5 (y1 - ȳ) 5.240 (x2 - x̄) 142.5 (y2 - ȳ) 2.004 (x3 - x̄) 28.5 (y3 - ȳ) 0.390 (x4 - x̄) -85.5 (y4 - ȳ) -1.231 (x5 - x̄) -199.5 (y5 - ȳ) -2.829 (x6 - x̄) -256.5 (y6 - ȳ) -3.575arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forward
- The following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forwardIf we have leucine (2-amino-4-methylpentanoic acid), alanine (2-aminopropanoic acid) and phenylalanine (2-amino-3-phenylpropanoic acid), indicate the tripeptides that can be formed (use the abbreviated symbols Leu., Ala and Phe).arrow_forward
- Briefly state why trifluoroacetic acid is more acidic than acetic acid.arrow_forwardExplain why acid chlorides are more reactive than amides in reactions with nucleophiles.arrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 101.7 mL of a 0.3500M solution of piperidine (C5H10NH) with a 0.05700M solution of HClO4. The pK of piperidine is 2.89. Calculate the pH of the base solution after the chemist has added 682.9 mL of the HClO solution to it. 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO solution added. 4 Round your answer to 2 decimal places. pH = .11 00. 18 Ararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning