
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 7.4P
An
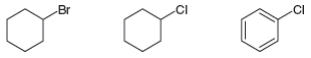
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
2. Propose a mechanism for this reaction.
ہلی سے ملی
N
H
(excess)
Steps and explanationn please.
Problem 5-48
Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C).
OH
H
OH
HO
CH2OH Ascorbic acid
O H
Problem 5-49
Assign R or S stereochemistry to the chirality centers in the following Newman projections:
H
Cl
H
CH3
H3C.
OH
H3C
(a)
H
H
H3C
(b)
CH3
H
Problem 5-52
Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each:
OH
OH
(a)
CH3CHCH2CH2CHCH3
CH3
H3C.
-OH
(c) H3C
CH3
(b)
Problem 5-66
Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed
antibiotic in the United States.
H2N H
IHH
S
Cephalexin
N.
CH3
CO₂H
Chapter 7 Solutions
ORGANIC CHEMISTRY
Ch. 7 - Problem 7.1 Telfairine, a naturally occurring...Ch. 7 - Give the IUPAC name for each compound. a. b. c. d.Ch. 7 - Prob. 7.3PCh. 7 - An sp3 hybridized CCl bond is more polar than an...Ch. 7 - Prob. 7.5PCh. 7 - Problem 7.6 Identify the nucleophile and leaving...Ch. 7 - Prob. 7.7PCh. 7 - Prob. 7.8PCh. 7 - What neutral nucleophile is needed to convert...Ch. 7 - Prob. 7.10P
Ch. 7 - Prob. 7.11PCh. 7 - Does the equilibrium favor the reactants or...Ch. 7 - Prob. 7.13PCh. 7 - Classify each solvent as protic or aprotic. a. b....Ch. 7 - Prob. 7.15PCh. 7 - Prob. 7.16PCh. 7 - Prob. 7.17PCh. 7 - Prob. 7.18PCh. 7 - Prob. 7.19PCh. 7 - Draw the product of each SN2 reaction and indicate...Ch. 7 - Prob. 7.21PCh. 7 - Prob. 7.22PCh. 7 - What happens to the rate of an SN1 reaction under...Ch. 7 - Draw the products of each SN1 reaction and...Ch. 7 - Classify each carbocation as 1,2, or 3. a. b. c....Ch. 7 - Prob. 7.26PCh. 7 - Prob. 7.27PCh. 7 - Prob. 7.28PCh. 7 - Prob. 7.29PCh. 7 - Problem 7.30 For each alkyl halide and...Ch. 7 - Prob. 7.31PCh. 7 - Prob. 7.32PCh. 7 - Prob. 7.33PCh. 7 - Prob. 7.34PCh. 7 - Prob. 7.35PCh. 7 - Prob. 7.36PCh. 7 - Prob. 7.37PCh. 7 - Prob. 7.38PCh. 7 - Prob. 7.39PCh. 7 - Give the IUPAC name for each compound, including...Ch. 7 - Draw the products formed when each alkyl halide is...Ch. 7 - Give the IUPAC name for each compound. a. c. e. b....Ch. 7 - Prob. 7.43PCh. 7 - Draw the eight constitutional isomers having the...Ch. 7 - Which compound in each pair has the higher boiling...Ch. 7 - Draw the products of each nucleophilic...Ch. 7 - Prob. 7.47PCh. 7 - Rank the species in each group in order of...Ch. 7 - Which of the following nucleophilic substitution...Ch. 7 - Rank the species in each group in order of...Ch. 7 - Prob. 7.51PCh. 7 - Prob. 7.52PCh. 7 - 7.53 Consider the following reaction.
Draw a...Ch. 7 - Prob. 7.54PCh. 7 - Draw the products of each SN2 reaction and...Ch. 7 - Prob. 7.56PCh. 7 - Prob. 7.57PCh. 7 - Consider the following SN1 reaction. a.Draw a...Ch. 7 - 7.59 Pick the reactant or solvent in each part...Ch. 7 - Draw the products of each SN1 reaction and...Ch. 7 - Prob. 7.61PCh. 7 - Prob. 7.62PCh. 7 - Prob. 7.63PCh. 7 - Fluticasone, the chapter-opening molecule, can be...Ch. 7 - Prob. 7.65PCh. 7 - 7.66 Diphenhydramine, the antihistamine in...Ch. 7 - Draw a stepwise, detailed mechanism for the...Ch. 7 - When a single compound contains both a nucleophile...Ch. 7 - Prob. 7.69PCh. 7 - Prob. 7.70PCh. 7 - Draw a stepwise, detailed mechanism f or the...Ch. 7 - Prob. 7.72PCh. 7 - Fill in the appropriate reagent or starting...Ch. 7 - Devise a synthesis of each compound from an alkyl...Ch. 7 - Suppose you have compounds A-D at y our disposal....Ch. 7 - Muscalure, the sex pheromone of the common...Ch. 7 - Prob. 7.77PCh. 7 - Prob. 7.78PCh. 7 - Draw a stepwise mechanism for the following...Ch. 7 - 7.80 As we will learn in Chapter 9, an epoxide is...Ch. 7 - Prob. 7.81PCh. 7 - In some nucleophilic substitutions under SN1...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- "Water gas" is an industrial fuel composed of a mixture of carbon monoxide and hydrogen gases. When this fuel is burned, carbon dioxide and water result. From the information given below, write a balanced equation and determine the enthalpy of this reaction: CO(g) + O2(g) → CO₂(g) + 282.8 kJ H2(g) + O2(g) → H₂O(g) + 241.8 kJ MacBook Airarrow_forwardPage of 3 4. Calculate AG for the following reaction at 25°C. Will the reaction occur (be spontaneous)? How do you know? NH3(g) + HCl(g) → NH4Cl(s) AH=-176.0 kJ AS-284.8 J-K-1arrow_forwardtrue or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 5. 4NO2(g) ⇔ 2N2O4(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY