
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 7.66P
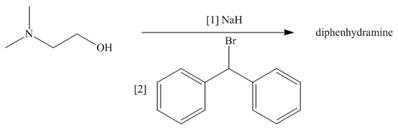
Diphenhydramine, the antihistamine in Benadryl, can be prepared by the following two-step sequence. What is the structure of diphenhydramine?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
If we assume a system with an anodic overpotential, the variation of n as a function
of current density:
1. at low fields is linear 2. at higher fields, it follows Tafel's law
Obtain the range of current densities for which the overpotential has the same value
when calculated for 1 and 2 cases (maximum relative difference of 5% compared to
the behavior for higher fields).
To which overpotential range does this correspond?
Data: i = 1.5 mA cm², T = 300°C, B = 0.64, R = 8.314 J K1 mol-1 and F = 96485 C mol-1.
Answer by equation please
Some of the theories used to describe interface structure can be distinguished by:1. the measured potential difference.2. the distribution of ions in solution.3. the calculation of charge density.4. the external Helmoltz plane.
Chapter 7 Solutions
ORGANIC CHEMISTRY
Ch. 7 - Problem 7.1 Telfairine, a naturally occurring...Ch. 7 - Give the IUPAC name for each compound. a. b. c. d.Ch. 7 - Prob. 7.3PCh. 7 - An sp3 hybridized CCl bond is more polar than an...Ch. 7 - Prob. 7.5PCh. 7 - Problem 7.6 Identify the nucleophile and leaving...Ch. 7 - Prob. 7.7PCh. 7 - Prob. 7.8PCh. 7 - What neutral nucleophile is needed to convert...Ch. 7 - Prob. 7.10P
Ch. 7 - Prob. 7.11PCh. 7 - Does the equilibrium favor the reactants or...Ch. 7 - Prob. 7.13PCh. 7 - Classify each solvent as protic or aprotic. a. b....Ch. 7 - Prob. 7.15PCh. 7 - Prob. 7.16PCh. 7 - Prob. 7.17PCh. 7 - Prob. 7.18PCh. 7 - Prob. 7.19PCh. 7 - Draw the product of each SN2 reaction and indicate...Ch. 7 - Prob. 7.21PCh. 7 - Prob. 7.22PCh. 7 - What happens to the rate of an SN1 reaction under...Ch. 7 - Draw the products of each SN1 reaction and...Ch. 7 - Classify each carbocation as 1,2, or 3. a. b. c....Ch. 7 - Prob. 7.26PCh. 7 - Prob. 7.27PCh. 7 - Prob. 7.28PCh. 7 - Prob. 7.29PCh. 7 - Problem 7.30 For each alkyl halide and...Ch. 7 - Prob. 7.31PCh. 7 - Prob. 7.32PCh. 7 - Prob. 7.33PCh. 7 - Prob. 7.34PCh. 7 - Prob. 7.35PCh. 7 - Prob. 7.36PCh. 7 - Prob. 7.37PCh. 7 - Prob. 7.38PCh. 7 - Prob. 7.39PCh. 7 - Give the IUPAC name for each compound, including...Ch. 7 - Draw the products formed when each alkyl halide is...Ch. 7 - Give the IUPAC name for each compound. a. c. e. b....Ch. 7 - Prob. 7.43PCh. 7 - Draw the eight constitutional isomers having the...Ch. 7 - Which compound in each pair has the higher boiling...Ch. 7 - Draw the products of each nucleophilic...Ch. 7 - Prob. 7.47PCh. 7 - Rank the species in each group in order of...Ch. 7 - Which of the following nucleophilic substitution...Ch. 7 - Rank the species in each group in order of...Ch. 7 - Prob. 7.51PCh. 7 - Prob. 7.52PCh. 7 - 7.53 Consider the following reaction.
Draw a...Ch. 7 - Prob. 7.54PCh. 7 - Draw the products of each SN2 reaction and...Ch. 7 - Prob. 7.56PCh. 7 - Prob. 7.57PCh. 7 - Consider the following SN1 reaction. a.Draw a...Ch. 7 - 7.59 Pick the reactant or solvent in each part...Ch. 7 - Draw the products of each SN1 reaction and...Ch. 7 - Prob. 7.61PCh. 7 - Prob. 7.62PCh. 7 - Prob. 7.63PCh. 7 - Fluticasone, the chapter-opening molecule, can be...Ch. 7 - Prob. 7.65PCh. 7 - 7.66 Diphenhydramine, the antihistamine in...Ch. 7 - Draw a stepwise, detailed mechanism for the...Ch. 7 - When a single compound contains both a nucleophile...Ch. 7 - Prob. 7.69PCh. 7 - Prob. 7.70PCh. 7 - Draw a stepwise, detailed mechanism f or the...Ch. 7 - Prob. 7.72PCh. 7 - Fill in the appropriate reagent or starting...Ch. 7 - Devise a synthesis of each compound from an alkyl...Ch. 7 - Suppose you have compounds A-D at y our disposal....Ch. 7 - Muscalure, the sex pheromone of the common...Ch. 7 - Prob. 7.77PCh. 7 - Prob. 7.78PCh. 7 - Draw a stepwise mechanism for the following...Ch. 7 - 7.80 As we will learn in Chapter 9, an epoxide is...Ch. 7 - Prob. 7.81PCh. 7 - In some nucleophilic substitutions under SN1...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?arrow_forwardUsing the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forwardIndicate characteristics of oxodec acid.arrow_forward
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License