
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 7.79P
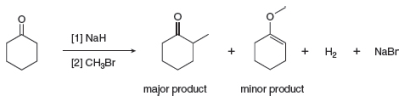
Draw a stepwise mechanism for the following reaction sequence.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Choose the best reagent(s) for carrying out the following conversions from the list below. Place the
letter corresponding to the best choice in the blank to the left of the conversion.
a.
KMnO4, H3O+
b. Tollens' Reagent [oxidizing reagent]
C.
NaBH4, ethanol
d.
1. BH3 2. H3O+
e.
1. CH3MgBr, ether 2. H3O+
f.
CrO3, H2SO4, H₂O
g. 1. Mg, ether 2. CO2
3. H3O+
h.
1. NaCN 2. H2SO4, H2, heat
i.
O3, then Zn and HOAC
j.
CH₂I
A.
B.
C.
CH CH=CHCH2COOH
Br
CEN
CH COOH + HOOCCH COOH
COOH
010
CH3
Draw the structures for each of the intermediates in the boxes provided for the synthesis below.
OCH3
Fe HO
HNO
(CHOO
pynding
H₂504
LHNO2
NACH-I
Fa
H₂O
HCL
HNO
180
Provide structure(s) for the starting material(s), reagent(s) or the major organic product(s) of each of the
following reactions or sequences of reactions. Show all relevant stereochemistry [three only]
A.
o
11
(CH3)CH — C— C
ether
(CH3)2CH-C-O-C-CH3
B.
CH3
CHy
CI
Staf
OH
C.
HC
OCHS
+
H₂O
Chapter 7 Solutions
ORGANIC CHEMISTRY
Ch. 7 - Problem 7.1 Telfairine, a naturally occurring...Ch. 7 - Give the IUPAC name for each compound. a. b. c. d.Ch. 7 - Prob. 7.3PCh. 7 - An sp3 hybridized CCl bond is more polar than an...Ch. 7 - Prob. 7.5PCh. 7 - Problem 7.6 Identify the nucleophile and leaving...Ch. 7 - Prob. 7.7PCh. 7 - Prob. 7.8PCh. 7 - What neutral nucleophile is needed to convert...Ch. 7 - Prob. 7.10P
Ch. 7 - Prob. 7.11PCh. 7 - Does the equilibrium favor the reactants or...Ch. 7 - Prob. 7.13PCh. 7 - Classify each solvent as protic or aprotic. a. b....Ch. 7 - Prob. 7.15PCh. 7 - Prob. 7.16PCh. 7 - Prob. 7.17PCh. 7 - Prob. 7.18PCh. 7 - Prob. 7.19PCh. 7 - Draw the product of each SN2 reaction and indicate...Ch. 7 - Prob. 7.21PCh. 7 - Prob. 7.22PCh. 7 - What happens to the rate of an SN1 reaction under...Ch. 7 - Draw the products of each SN1 reaction and...Ch. 7 - Classify each carbocation as 1,2, or 3. a. b. c....Ch. 7 - Prob. 7.26PCh. 7 - Prob. 7.27PCh. 7 - Prob. 7.28PCh. 7 - Prob. 7.29PCh. 7 - Problem 7.30 For each alkyl halide and...Ch. 7 - Prob. 7.31PCh. 7 - Prob. 7.32PCh. 7 - Prob. 7.33PCh. 7 - Prob. 7.34PCh. 7 - Prob. 7.35PCh. 7 - Prob. 7.36PCh. 7 - Prob. 7.37PCh. 7 - Prob. 7.38PCh. 7 - Prob. 7.39PCh. 7 - Give the IUPAC name for each compound, including...Ch. 7 - Draw the products formed when each alkyl halide is...Ch. 7 - Give the IUPAC name for each compound. a. c. e. b....Ch. 7 - Prob. 7.43PCh. 7 - Draw the eight constitutional isomers having the...Ch. 7 - Which compound in each pair has the higher boiling...Ch. 7 - Draw the products of each nucleophilic...Ch. 7 - Prob. 7.47PCh. 7 - Rank the species in each group in order of...Ch. 7 - Which of the following nucleophilic substitution...Ch. 7 - Rank the species in each group in order of...Ch. 7 - Prob. 7.51PCh. 7 - Prob. 7.52PCh. 7 - 7.53 Consider the following reaction.
Draw a...Ch. 7 - Prob. 7.54PCh. 7 - Draw the products of each SN2 reaction and...Ch. 7 - Prob. 7.56PCh. 7 - Prob. 7.57PCh. 7 - Consider the following SN1 reaction. a.Draw a...Ch. 7 - 7.59 Pick the reactant or solvent in each part...Ch. 7 - Draw the products of each SN1 reaction and...Ch. 7 - Prob. 7.61PCh. 7 - Prob. 7.62PCh. 7 - Prob. 7.63PCh. 7 - Fluticasone, the chapter-opening molecule, can be...Ch. 7 - Prob. 7.65PCh. 7 - 7.66 Diphenhydramine, the antihistamine in...Ch. 7 - Draw a stepwise, detailed mechanism for the...Ch. 7 - When a single compound contains both a nucleophile...Ch. 7 - Prob. 7.69PCh. 7 - Prob. 7.70PCh. 7 - Draw a stepwise, detailed mechanism f or the...Ch. 7 - Prob. 7.72PCh. 7 - Fill in the appropriate reagent or starting...Ch. 7 - Devise a synthesis of each compound from an alkyl...Ch. 7 - Suppose you have compounds A-D at y our disposal....Ch. 7 - Muscalure, the sex pheromone of the common...Ch. 7 - Prob. 7.77PCh. 7 - Prob. 7.78PCh. 7 - Draw a stepwise mechanism for the following...Ch. 7 - 7.80 As we will learn in Chapter 9, an epoxide is...Ch. 7 - Prob. 7.81PCh. 7 - In some nucleophilic substitutions under SN1...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
What are the cervical and lumbar enlargements?
Principles of Anatomy and Physiology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the reaction sequence below to answer the following questions: EtO Compound X 1. NaOEt, EtOH OEt Br CO₂Et NaOEt, EtOH Compound Z CO₂Et Compound Y A. Compound X, diethyl propanedioate, is more commonly known as a. ethyl acetoacetate b. acetoacetic ester C. oxalic ester d. malonic ester 3. Write the complete stepwise mechanism for the conversion of Compound X into Compound Y. Show all electron flow with arrows and draw all intermediate structures.arrow_forwardClassify each of the following nitrogen atoms in the following compounds as primary, secondary, tertiary, or quaternary [three only] CH3 HO-CHCHNHCH3 A. B. C. H&C CH3 D. HO phedrine CH2CHCH3 amphetamine NH₂ mepiquat chloride faxofenadine OH H&C CH CO₂Harrow_forwardDraw the structure of the aldol self-condensation product for each of the following compounds. If a compound does not undergo aldol self-condensation, explain why it does not. A. B. CHICHCH₂OH CH3CHCH2CH CH3 CH3 C. CH 30 H3C-C-C-H CH3 questionsarrow_forward
- . A.Propose a synthesis for propylbenzene which avoids the problems of direct Friedel-Crafts alkylation. B. Consider the reaction below to answer the following questions. A B C NO2 Febr Brz D The Lewis acid catalyst in the reaction is: a. The nucleophile in the reaction is: b. C. d. This reaction proceeds Draw the structure of product D. (faster or slower) than benzene.arrow_forwardConsider the reaction below to answer the following questions. HOCH CHOH На A B C D H₂Oarrow_forwardConsider the structures below to answer the following questions. A. Indicate the most acidic hydrogens in each of the molecules. OH CH-H CH₂C-H H&C མིངྒཱའི B. Rank the molecules above in order of increasing acidity (least acidic to most acidic). a. III, II, I b. II, III, I C. I, II, III d. II, I, IIIarrow_forward
- Consider the reaction below to answer the following questions. H H+ A B CH₂OH 5% NaOCH, CH₂OH A. Which carbonyl compound functions as the electrophile in this reaction? B. Draw the structure of the enolate ion that is generated during the course of this reaction. C. This reaction is an example of: a. a mixed Claisen condensation. b. C. d. a Dieckman condensation. a Michael reaction. a mixed aldol reaction.arrow_forwardGive the major organic product(s) of each of the following reactions or sequences of reactions. Show all relevant stereochemistry. [two only] CH3O (11 HC-C-C-CH3 A. CH3 12. NaOHarrow_forwardDiethyl malonate can be prepared by the following reaction sequence. Draw the structures of each of the missing intermediates in the boxes provided. 17 1. Br PBr H&C OH 2 H₂O CH3CH₂OH На NaCN H₂SO4 NC. CH CH₂OH на H₂O, heat CH₂ OCHCH3 ཝསི། ཡིཀྑཱམུདྡྷནྟ CH₂ OEtarrow_forwardThe reaction of a carboxylic acid with an alcohol in the presence of acid is termed Fischer esterification. OH + CH₂OH На B C A. The nucleophile in this reaction is B. Compound C functions as a. a base scavenger b. a solvent C. a catalyst d. a neutralizer C. Fischer esterification is an example of: . a. nucleophilic acyl addition b. nucleophilic acyl substitution c. nucleophilic acyl elimination d. nucleophilic acyl rearrangement in this reaction.arrow_forwardA highly useful and general method for the synthesis of alcohols is the addition of Grignard reagents to carbonyl compounds. Show what Grignard reagent and what carbonyl compound you would start with to prepare each alcohol below. List all possibilities. OH C-CH2CH3 CH3arrow_forwardRank the following groups of compounds from most acidic (1) to least acidic (4). Place the number corresponding to the compound's relative rank in the blank below the structure. NO2 a. b. NO2 NO2 CH,CH,CH,CH,OH. CHCHCH-CHOH. CH-CH-CH,CH;OH CH-CHCH-CH-OH OH OH CH₂OH COH ဒီ ပုံ ပုံ H&C CN CN ĆNarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY