
Concept explainers
Interpretation:
The electron-poor H atom in methanol is to be identified, and the mechanism by which methanol acts as an acid in a proton transfer reaction with
Concept introduction:
An electron poor atom or a molecule is an atom or a site in a molecule that has fewer electrons than the number required for stability. In a proton transfer reaction, a proton is transferred from a Bronsted-Lowry acid (proton donor) to a Bronsted–Lowry base (proton acceptor). In an elementary step, electrons tend to flow from an electron-rich site to an electron-poor site. The curved arrow notation shows the movement of valence electrons.
Answer to Problem 7.1P
The electron-poor H atom in methanol is:
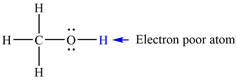
The mechanism by which methanol acts as an acid in a proton transfer reaction with
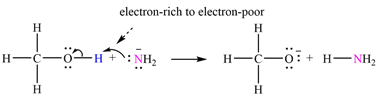
Explanation of Solution
Atoms in
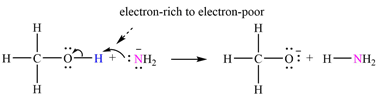
The curved arrows are drawn from the electron-rich site
Want to see more full solutions like this?
Chapter 7 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- О δα HO- H -Br δα HO-- + + -Br [B] 8+ HO- -Br δα नarrow_forward1/2 - 51% + » GAY Organic Reactions Assignment /26 Write the type of reaction that is occurring on the line provided then complete the reaction. Only include the major products and any byproducts (e.g. H₂O) but no minor products. Please use either full structural diagrams or the combination method shown in the lesson. Skeletal/line diagrams will not be accepted. H3C 1. 2. CH3 A Acid OH Type of Reaction: NH Type of Reaction: + H₂O Catalyst + HBr 3. Type of Reaction: H3C 4. Type Reaction: 5. H3C CH2 + H2O OH + [0] CH3 Type of Reaction: 6. OH CH3 HO CH3 + Type of Reaction: 7. Type of Reaction: + [H]arrow_forwardhumbnai Concentration Terms[1].pdf ox + New Home Edit Sign in Comment Convert Page Fill & Sign Protect Tools Batch +WPS A Free Trial Share Inter Concreting Concentration forms. Hydrogen peroxide is a powerful oxidizing agent wed in concentrated solution in rocket fuels and in dilute solution as a hair bleach. An aqueous sulation of H2O2 is 30% by mass and has density of #liligime calculat the Ⓒmolality ⑥mole fraction of molarity. 20 9. B. A sample of Commercial Concentrated hydrochloric ETarrow_forward
- If a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forward
- Draw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forwardChoose the right answerarrow_forward8. What is the major product of the following reaction? KMnO4 b a TOH OH OH C d OH "OH HO OH OHarrow_forward
- Choose the right answerarrow_forward3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


