
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 4Q
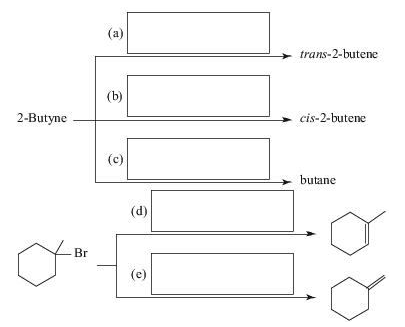
Supply the missing reagents.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton
transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted
without ambiguity.
a.
2.
1. LDA
3. H3O+
HO
b.
H3C CH3
H3O+
✓ H
OH
2. Provide reagents/conditions to accomplish the following syntheses. More than one step is
required in some cases.
a.
CH3
Chapter 7 Solutions
Organic Chemistry
Ch. 7 - Prob. 1PPCh. 7 - Prob. 2PPCh. 7 - Prob. 3PPCh. 7 - Prob. 4PPCh. 7 - Practice Problem 7.5
How many stereoisomers are...Ch. 7 - Prob. 6PPCh. 7 - Prob. 7PPCh. 7 - PRACTICE PROBLEM 7.8
Examine Solved Problem 7.3....Ch. 7 - Prob. 9PPCh. 7 - Practice Problem 7.10 When...
Ch. 7 - Practice Problem 7.11
(a) When...Ch. 7 - Practice Problem 7.14
Dehydration of 2-propanol...Ch. 7 - Practice Problem 7.15
Rank the following alcohols...Ch. 7 - Practice Problem 7.16
Acid-catalyzed dehydration...Ch. 7 - Practice Problem 7.17 Acid-catalyzed dehydration...Ch. 7 - Prob. 16PPCh. 7 - Prob. 17PPCh. 7 - Practice Problem 7.20
Show how you might...Ch. 7 - Prob. 19PPCh. 7 - Prob. 20PPCh. 7 - Practice Problem 7.23
Write the structure of...Ch. 7 - Prob. 22PPCh. 7 - Prob. 23PPCh. 7 - Practice Problem 7.26 (a) Devise retrosynthetic...Ch. 7 - Each of the following names is incorrect, Give the...Ch. 7 - Prob. 26PCh. 7 - Prob. 27PCh. 7 - Give the IUPAC names for each of the following:...Ch. 7 - Prob. 29PCh. 7 - Prob. 30PCh. 7 - Prob. 31PCh. 7 - Prob. 32PCh. 7 - 7.54. Outline a synthesis of phenylethyne from...Ch. 7 - Prob. 34PCh. 7 - Prob. 35PCh. 7 - Prob. 36PCh. 7 - Prob. 37PCh. 7 - 7.35. Write structural formulas for all the...Ch. 7 - 7.47. Starting with an appropriate alkyl halide...Ch. 7 - Arrange the following alcohols in order of their...Ch. 7 - Prob. 41PCh. 7 - Prob. 42PCh. 7 - Prob. 43PCh. 7 - Prob. 44PCh. 7 - 7.49. What is the index of hydrogen deficiency...Ch. 7 - Prob. 46PCh. 7 - Prob. 47PCh. 7 - Compounds I and J both have the molecular formula...Ch. 7 - Prob. 49PCh. 7 - Prob. 50PCh. 7 - Consider the interconversion of cis-2-butene and...Ch. 7 - Prob. 52PCh. 7 - (a) Using reactions studied in this chapter, show...Ch. 7 - Prob. 54PCh. 7 - Prob. 55PCh. 7 - 1. Write the structure(s) of the major product(s)...Ch. 7 - Prob. 2LGPCh. 7 - (a) Write the structure of the product(s) formed...Ch. 7 - Prob. 4LGPCh. 7 - Prob. 5LGPCh. 7 - 7.1 Which conditions/reagents would you employ to...Ch. 7 - Which of the following names is incorrect? (a)...Ch. 7 - 7.3 Select the major product of the reaction
Ch. 7 - 7.4 Supply the missing reagents.
Ch. 7 - Arrange the following alkenes in order of...Ch. 7 - 7.6 Complete the following synthesis.
Additional Science Textbook Solutions
Find more solutions based on key concepts
Sketch qualitatively the electric field lines both between and outside two concentric conducting spherical shel...
Fundamentals of Physics Extended
Modified True/False 3. __________ Aquatic microorganisms are more prevalent near the surface than at the bottom...
Microbiology with Diseases by Body System (5th Edition)
8. A classic way to isolate thymidylate synthase—negative mutants of bacteria is to treat a growing culture wit...
Biochemistry: Concepts and Connections (2nd Edition)
Sketch the following spectra that would be obtained for 2-chloroethanol: a. The 1H NMR spectrum for an anhydrou...
Organic Chemistry (8th Edition)
explain the function of fermentation and the conditions under which it occurs?
Biology: Life on Earth with Physiology (11th Edition)
3. In a test of his chromosome theory of heredity, Morgan crossed an F1 female Drosophila with red eyes to a m...
Genetic Analysis: An Integrated Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forward
- a. H3C CH3 H, 1.0 equiv. Br2arrow_forwardH3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forward
- in the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forwardIf we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY