
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 41P
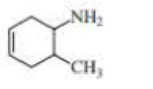
What is each compound’s systematic name?
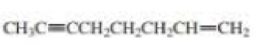
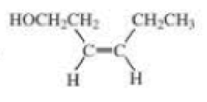
- a. CH3CH2C≡CCH2CH2C≡CH
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Help fix my arrows please
no Ai walkthroughs
Given the data attached, provide a drawing of the corresponding structure.
Chapter 7 Solutions
EBK ORGANIC CHEMISTRY
Ch. 7.1 - Prob. 1PCh. 7.1 - Name the following:Ch. 7.1 - What is the molecular formula for a monocyclic...Ch. 7.1 - Draw the condensed and skeletal structures for...Ch. 7.1 - Draw the structure and give the common and...Ch. 7.2 - Prob. 6PCh. 7.2 - Name the following:Ch. 7.3 - What orbitals are used to form the carbon-carbon ...Ch. 7.4 - Prob. 9PCh. 7.4 - Why does cis-2-butene have a higher boiling point...
Ch. 7.6 - Prob. 12PCh. 7.6 - Prob. 13PCh. 7.7 - Prob. 14PCh. 7.7 - Which alkyne should be used for the synthesis of...Ch. 7.7 - Prob. 16PCh. 7.8 - Prob. 17PCh. 7.8 - Only one alkyne forms an aldehyde when it...Ch. 7.9 - Describe the alkyne you should start with and the...Ch. 7.9 - Prob. 20PCh. 7.10 - Prob. 21PCh. 7.10 - Prob. 22PCh. 7.10 - Prob. 23PCh. 7.10 - Rank the following from strongest base to weakest...Ch. 7.10 - Prob. 26PCh. 7.12 - Prob. 28PCh. 7 - What is the major product obtained from the...Ch. 7 - Draw a condensed structure for each of the...Ch. 7 - A student was given the structural formula of...Ch. 7 - Prob. 32PCh. 7 - What is each compounds systematic name?Ch. 7 - What reagents should be used to carry out the...Ch. 7 - Prob. 35PCh. 7 - Draw the mechanism for the following reaction:Ch. 7 - Prob. 37PCh. 7 - Prob. 38PCh. 7 - What is the major product of the reaction of 1 mol...Ch. 7 - Answer Problem 39, parts a-b, using 2-butyne as...Ch. 7 - What is each compounds systematic name? a....Ch. 7 - What is the molecular formula of a hydrocarbon...Ch. 7 - a. Starting with 3-methyl 1-butyne, how can you...Ch. 7 - Prob. 44PCh. 7 - Which of the following pairs are keto-enol...Ch. 7 - Prob. 46PCh. 7 - Do the equilibria of the following acid-base...Ch. 7 - What is each compounds systematic name?Ch. 7 - What stereoisomers are obtained when 2-butyne...Ch. 7 - Prob. 50PCh. 7 - Draw the keto tautomer for each of the following:Ch. 7 - Show how each of the following compounds can be...Ch. 7 - A chemist is planning to synthesize 3-octyne by...Ch. 7 - Prob. 54PCh. 7 - What stereoisomers are obtained from the following...Ch. 7 - Prob. 56PCh. 7 - Prob. 57PCh. 7 - Prob. 58PCh. 7 - Show how the following compound can be prepared...Ch. 7 - Prob. 60P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help 20arrow_forwardProvide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forward
- 3) Draw a detailed mechanism and predict the product of the reaction shown? 1) EtMgBr 2) H3O+arrow_forwardHow to draw the mechanism for this reaction?arrow_forward> H₂C=C-CH2-CH3 B. H₂O Pt C. + H2 + H₂O H D. 16. Give the IUPAC name for each of the following: B. Cl Cl c. Cl Cl 17. Draw the line-angle formula for each of the following compounds: 1. phenol 2. 1,3-dichlorobenzene 3. 4-ethyltoluene < Previous Submit Assignment Next ▸arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY