
Concept explainers
(a)
Interpretation:
The E1 product for the following reaction should be written.
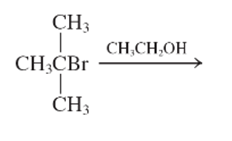
Concept Introduction:
A type of organic reaction in which two substituents get eliminated from a given molecule by one step mechanism or two step mechanism is known as elimination reaction. The reaction which occurs in one step is known as E2 reaction whereas the reaction which occurs in two steps is known as E1 reaction.
In E1 reaction, elimination of HX substituent takes place and produces a double bond. It is a unimolecular reaction.
(b)
Interpretation:
The E1 product for the following reaction should be written.
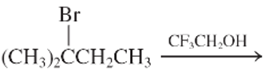
Concept Introduction:
A type of organic reaction in which two substituents get eliminated from a given molecule by one step mechanism or two step mechanism is known as elimination reaction. The reaction which occurs in one step is known as E2 reaction whereas the reaction which occurs in two steps is known as E1 reaction.
In E1 reaction, elimination of HX substituent takes place and produces a double bond. It is a unimolecular reaction.
(c)
Interpretation:
The E1 product for the following reaction should be written.
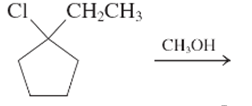
Concept Introduction:
A type of organic reaction in which two substituents get eliminated from a given molecule by one step mechanism or two step mechanism is known as elimination reaction. The reaction which occurs in one step is known as E2 reaction whereas the reaction which occurs in two steps is known as E1 reaction.
In E1 reaction, elimination of HX substituent takes place and produces a double bond. It is a unimolecular reaction.
(d)
Interpretation:
The E1 product for the following reaction should be written.
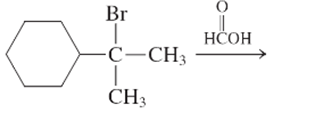
Concept Introduction:
A type of organic reaction in which two substituents get eliminated from a given molecule by one step mechanism or two step mechanism is known as elimination reaction. The reaction which occurs in one step is known as E2 reaction whereas the reaction which occurs in two steps is known as E1 reaction.
In E1 reaction, elimination of HX substituent takes place and produces a double bond. It is a unimolecular reaction.
(e)
Interpretation:
The E1 product for the following reaction should be written.
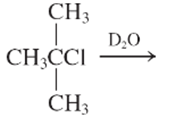
Concept Introduction:
A type of organic reaction in which two substituents get eliminated from a given molecule by one step mechanism or two step mechanism is known as elimination reaction. The reaction which occurs in one step is known as E2 reaction whereas the reaction which occurs in two steps is known as E1 reaction.
In E1 reaction, elimination of HX substituent takes place and produces a double bond. It is a unimolecular reaction.
(f)
Interpretation:
The E1 product for the following reaction should be written.
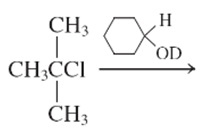
Concept Introduction:
A type of organic reaction in which two substituents get eliminated from a given molecule by one step mechanism or two step mechanism is known as elimination reaction. The reaction which occurs in one step is known as E2 reaction whereas the reaction which occurs in two steps is known as E1 reaction.
In E1 reaction, elimination of HX substituent takes place and produces a double bond. It is a unimolecular reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: Structure and Function
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

