
(a)
Interpretation: The products formed due to elimination and most likely mechanism for below reactionsshouldbe written.
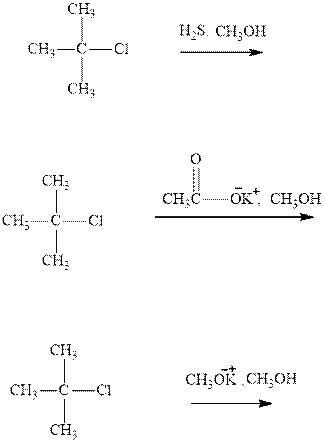
Concept introduction: Carbocations generated from
If the leaving group present eliminates along with a proton in absence of any strong nucleophile the double bond formation might occur. Such reactions are known as two step unimolecuar elimination, abbreviated as
(b)
Interpretation: The rates of below three reactions should be compared.
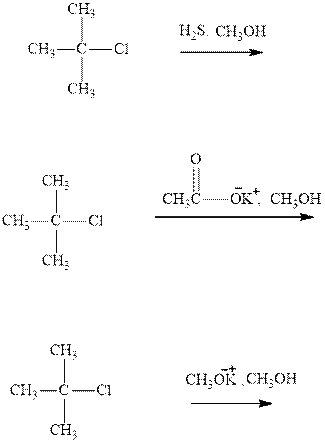
Concept introduction: Carbocations generated from alkyl halides have two fates; they can be either trapped by nucleophiles to give substitution product or may deprotonate to yield a small amount of alkene.
If the leaving group present eliminates along with a proton in absence of any strong nucleophile the double bond formation might occur. Such reactions are known as two step unimolecuar elimination, abbreviated as
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: Structure and Function
- Write the systematic name of each organic molecule: structure i HO OH Explanation Check name ☐ ☐arrow_forwardX 5 Check the box under each molecule that has a total of five ẞ hydrogens. If none of the molecules fit this description, check the box underneath the table. CI Br Br Br 0 None of these molecules have a total of five ẞ hydrogens. Explanation Check esc F1 F2 tab caps lock fn Q @2 A W # 3 OH O OH HO © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility IK F7 F7 F8 TA F9 F10 & 6 28 * ( > 7 8 9 0 80 F3 O F4 KKO F5 F6 S 64 $ D % 25 R T Y U பட F G H O J K L Z X C V B N M H control option command P H F11 F12 + || { [ command optionarrow_forwardAn open vessel containing water stands in a laboratory measuring 5.0 m x 5.0 m x 3.0 m at 25 °C ; the vapor pressure (vp) of water at this temperature is 3.2 kPa. When the system has come to equilibrium, what mass of water will be found in the air if there is no ventilation? Repeat the calculation for open vessels containing benzene (vp = 13.1 kPa) and mercury (vp = 0.23 Pa)arrow_forward
- Every chemist knows to ‘add acid to water with constant stirring’ when diluting a concentrated acid in order to keep the solution from spewing boiling acid all over the place. Explain how this one fact is enough to prove that strong acids and water do not form ideal solutions.arrow_forwardThe predominant components of our atmosphere are N₂, O₂, and Ar in the following mole fractions: χN2 = 0.780, χO2 = 0.21, χAr = 0.01. Assuming that these molecules act as ideal gases, calculate ΔGmix, ΔSmix, and ΔHmix when the total pressure is 1 bar and the temperature is 300 K.arrow_forwarddG = Vdp - SdT + μA dnA + μB dnB + ... so that under constant pressure and temperature conditions, the chemical potential of a component is the rate of change of the Gibbs energy of the system with respect to changing composition, μJ = (∂G / ∂nJ)p,T,n' Using first principles prove that under conditions of constant volume and temperature, the chemical potential is a measure of the partial molar Helmholtz energy (μJ = (∂A / ∂nJ)V,T,n')arrow_forward
- The vapor pressure of dichloromethane at 20.0 °C is 58.0 kPa and its enthalpy of vaporization is 32.7 kJ/mol. Estimate the temperature at which its vapor pressure is 66.0 kPa.arrow_forwardDraw the structure of A, the minor E1 product of the reaction. Cl Skip Part Check F1 esc CH_CH OH, D 3 2 Click and drag to start drawing a structure. 80 R3 F4 F2 F3 @ 2 # $ 4 3 Q W 95 % KO 5 F6 A F7 × G ☐ Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ►II A A F8 F9 F10 FL 6 7 88 & * 8 9 LLI E R T Y U A S D lock LL F G H 0 P J K L Z X C V B N M 9 Harrow_forwardFrom the choices given, which two substances have the same crystal structure? (Select both) Group of answer choices ZnS (zincblende) Diamond TiO2 (rutile) ZnS (wurtzite)arrow_forward
- Potassium (K) blends with germanium (Ge) to form a Zintl phase with a chemical formula of K4Ge4. Which of the following elements would you expect potassium to blend with to form an alloy? Electronegativities: As (2.0), Cl (3.0), Ge (1.8), K (0.8), S (2.5), Ti (1.5) Group of answer choices Arsenic (As) Sulfur (S) Chlorine (Cl) Titanium (Ti)arrow_forwardConsider two elements, X and Z. Both have cubic-based unit cells with the same edge lengths. X has a bcc unit cell while Z has a fcc unit cell. Which of the following statements is TRUE? Group of answer choices Z has a larger density than X X has more particles in its unit cell than Z does X has a larger density than Z Z has a larger unit cell volume than Xarrow_forwardHow many particles does a face-centered cubic (fcc) unit cell contain? Group of answer choices 2 14 8 4arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





