
Interpretation: Structures should be assigned to various possible isomeric bromoalkanes with the molecular formula
Concept introduction: Molecules that possess identical molecular formula but vary in the manner of their connectivity of the bonds are referred to as structural isomers. These are essentially found in
For instance, the smallest branched alkane possible is
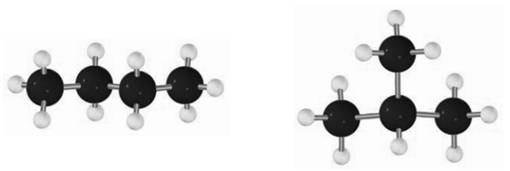
The two compounds, in fact, constitute a pair of constitutional isomers. These kinds of isomers are formed by cleavage and replacement of bonds, unlike the conformational isomers that result due to rotation around the bond.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
ORGANIC CHEMISTRY (LL)-PACKAGE
- Can you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forward
- consider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
- What is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forward
- Predict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
