
Concept explainers
Using your model of butane
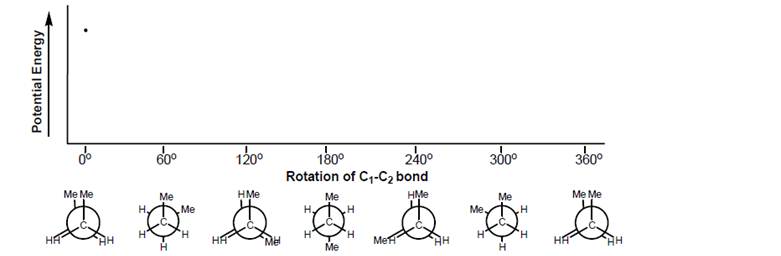 a. Label each Newman projection of butane on the graph with the words staggered, eclipsed, gauche, and anti, as appropriate. (Note that some structures will have more than one label.)
a. Label each Newman projection of butane on the graph with the words staggered, eclipsed, gauche, and anti, as appropriate. (Note that some structures will have more than one label.)
b. Draw a wedge and dash bond representation of butane in its lowest P.E. conformation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Custom eBook for Organic Chemistry
- Nonearrow_forwardTransmitance 3. Which one of the following compounds corresponds to this IR spectrum? Point out the absorption band(s) that helped you decide. OH H3C OH H₂C CH3 H3C CH3 H3C INFRARED SPECTRUM 0.8- 0.6 0.4- 0.2 3000 2000 1000 Wavenumber (cm-1) 4. Consider this compound: H3C On the structure above, label the different types of H's as A, B, C, etc. In table form, list the labeled signals, and for each one state the number of hydrogens, their shifts, and the splitting you would observe for these hydrogens in the ¹H NMR spectrum. Label # of hydrogens splitting Shift (2)arrow_forwardNonearrow_forward
- Draw the Lewis structure of C2H4Oarrow_forwarda) 5. Circle all acidic (and anticoplanar to the Leaving group) protons in the following molecules, Solve these elimination reactions, and identify the major and minor products where appropriate: 20 points + NaOCH3 Br (2 productarrow_forwardNonearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
