
Custom eBook for Organic Chemistry
2nd Edition
ISBN: 9798214171104
Author: Straumanis
Publisher: Cengage Custom
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 6, Problem 1CTQ
Interpretation Introduction
Interpretation:
The model of butane should be constructed.
Concept Introduction:
The spatial arrangement of atoms in a molecule obtained by rotation around carbon - carbon single bond is known as conformation.
Expert Solution & Answer
Explanation of Solution
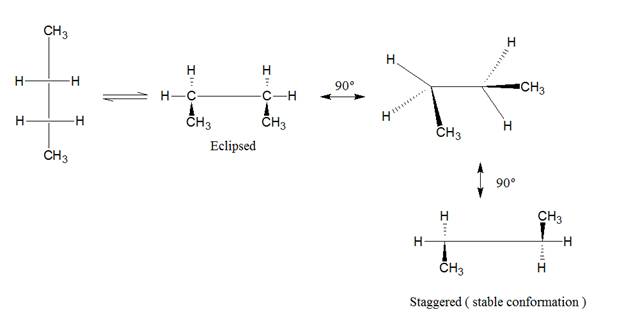
In Newman conformation, the first carbon is represented by dot whereas the back carbon is represented by circle.
Staggered and eclipsed are the two type of conformation in Newman projection.
The molecule of butane can be represented by sawhorse, Newman projection, and wedge dash representation as follows:
The molecule of butane can be viewed from
represented as follows:
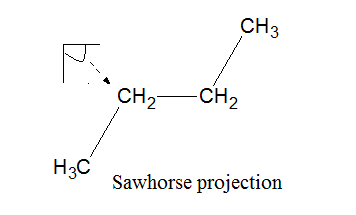
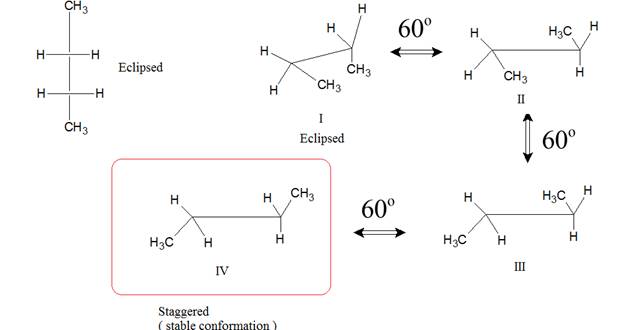
The representation of butane by wedge and dash representation is given as follows:
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
How many signals do you expect in the H NMR spectrum for this molecule?
Br
Br
Write the answer below.
Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H
atoms that would contribute to the same signal as the H already highlighted red.
Note for advanced students: In this question, any multiplet is counted as one signal.
Number of signals in the 'H NMR spectrum.
For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to
the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
No additional Hs to color in top
molecule
For the molecule in the bottom drawing area, highlight in red any other H atoms that will
contribute to the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
No additional Hs to color in bottom
molecule
In the drawing area below, draw the major products of this organic reaction:
1. NaOH
?
2. CH3Br
If there are no major products, because nothing much will happen to the reactant under these reaction conditions, check the box under the drawing area
instead.
No reaction.
Click and drag to start drawing a
structure.
☐ : A
ค
Predict the major products of the following organic reaction:
NC
Δ
?
Some important Notes:
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
• Be sure to draw bonds carefully to show important geometric relationships between substituents.
Note: if your answer contains a complicated ring structure, you must use one of the molecular fragment stamps (available in the menu at right) to enter the
ring structure. You can add any substituents using the pencil tool in the usual way.
Click and drag to start drawing a
structure.
Х
а
Chapter 6 Solutions
Custom eBook for Organic Chemistry
Ch. 6 - Prob. 1CTQCh. 6 - Prob. 2CTQCh. 6 - Prob. 4CTQCh. 6 - Prob. 5CTQCh. 6 - Complete this graph of relative potential energy...Ch. 6 - Prob. 7CTQCh. 6 - Prob. 8CTQCh. 6 - Prob. 9CTQCh. 6 - Consider the Newman projection below. a. Draw a...Ch. 6 - Draw a Newman projection showing the lowest P.E....
Ch. 6 - Prob. 12CTQCh. 6 - Prob. 13CTQCh. 6 - In skeletal representations the hydrogens are not...Ch. 6 - Prob. 15CTQCh. 6 - Prob. 16CTQCh. 6 - Prob. 17CTQCh. 6 - Prob. 19CTQCh. 6 - Prob. 20CTQCh. 6 - Prob. 21CTQCh. 6 - Prob. 22CTQCh. 6 - Prob. 23CTQCh. 6 - Draw a constitutional isomer of pentane,...Ch. 6 - How many H’s are lost from the molecular formula...Ch. 6 - How many ifs are lost from the molecular formula...Ch. 6 - Prob. 27CTQCh. 6 - What is the degree of unsaturation for the example...Ch. 6 - Without counting hydrogens, determine which one of...Ch. 6 - Determine the degree of unsaturation (and draw a...Ch. 6 - a model of each molecule shown above: Is the...Ch. 6 - Prob. 32CTQCh. 6 - Prob. 33CTQCh. 6 - Label each double bond E, Z, or neither. (It may...Ch. 6 - Prob. 35CTQCh. 6 - Prob. 36CTQCh. 6 - Indicate the relationship between each pair....Ch. 6 - Prob. 38CTQCh. 6 - Prob. 1ECh. 6 - Prob. 2ECh. 6 - Using your model of butane (CH3CH2CH2CH3) ,...Ch. 6 - Consider the molecule 1-bromo-2-methylbutane. C3...Ch. 6 - Prob. 5ECh. 6 - Prob. 8ECh. 6 - Prob. 9ECh. 6 - Prob. 10ECh. 6 - Prob. 11ECh. 6 - Prob. 12ECh. 6 - Prob. 13ECh. 6 - Prob. 15ECh. 6 - Prob. 16ECh. 6 - Prob. 17ECh. 6 - Prob. 18ECh. 6 - Prob. 19ECh. 6 - Prob. 20ECh. 6 - Prob. 21ECh. 6 - Double bonds do not rotate freely under normal...Ch. 6 - up an example (not appearing in this ChemActivity)...Ch. 6 - Prob. 24ECh. 6 - Prob. 25E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major products of this organic reaction. Be sure you use dash and wedge bonds to show stereochemistry where it's important. + ☑ OH 1. TsCl, py .... 文 P 2. t-BuO K Click and drag to start drawing a structure.arrow_forwardConsider this organic reaction: ( Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. Click and drag to start drawing a structure. Х : а ค 1arrow_forwardIn the drawing area below, draw the major products of this organic reaction: If there are no major products, because nothing much will happen to the reactant under these reaction conditions, check the box under the drawing area instead. 1. NaH 2. CH3Br ? Click and drag to start drawing a structure. No reaction. : ☐ Narrow_forward
- + Predict the major product of the following reaction. : ☐ + ☑ ค OH H₂SO4 Click and drag to start drawing a structure.arrow_forwardConsider this organic reaction: ... OH CI Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. ☐ No Reaction. Click and drag to start drawing a structure. : аarrow_forwardConsider the following reactants: Br Would elimination take place at a significant rate between these reactants? Note for advanced students: by significant, we mean that the rate of elimination would be greater than the rate of competing substitution reactions. yes O no If you said elimination would take place, draw the major products in the upper drawing area. If you said elimination would take place, also draw the complete mechanism for one of the major products in the lower drawing area. If there is more than one major product, you may draw the mechanism that leads to any of them. Major Products:arrow_forward
- Draw one product of an elimination reaction between the molecules below. Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side products of the reaction. OH + ! : ☐ + Х Click and drag to start drawing a structure.arrow_forwardFind one pertinent analytical procedure for each of following questions relating to food safety analysis. Question 1: The presence of lead, mercury and cadmium in canned tuna Question 2: Correct use of food labellingarrow_forwardFormulate TWO key questions that are are specifically in relation to food safety. In addition to this, convert these questions into a requirement for chemical analysis.arrow_forward
- What are the retrosynthesis and forward synthesis of these reactions?arrow_forwardWhich of the given reactions would form meso product? H₂O, H2SO4 III m CH3 CH₂ONa CH3OH || H₂O, H2SO4 CH3 1. LiAlH4, THF 2. H₂O CH3 IVarrow_forwardWhat is the major product of the following reaction? O IV III HCI D = III ა IVarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning