
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5.SE, Problem 66AP
Interpretation Introduction
Interpretation:
R or S configuration is to be assigned to Cephalaxin.
Concept introduction:
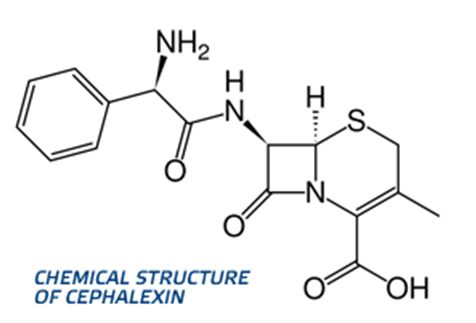
Cefalexin, also spelled cephalexin, is an antibiotic that can treat a number of bacterial infections. It kills gram-positive and some gram-negative bacteria by disrupting the growth of the bacterial cell wall.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
can someone give a description of this NMR including whether its a triplt singlet doublet where the peak is around at ppm and what functional group it represents
1. Determine the relationship between the following molecules as identical, diastereomers, or enantiomers (6
points, 2 points each).
OH
OH
OH
A-A
OH
HOT
HO-
ACHN
and
HO-
ACHN
OH
HO
HO
°
OH
and
OH
OH
SH
and
...SH
20,0
Complete the electron pushing mechanism to
y drawing the necomery unicaciones and carved on for
Step 1: Add curved arms for the tint step, traiment with NalilĻ. The Nation
458
Step 2: Added for the second step, inalment with), how the "counterion
bar
Step 3: Daw the products of the last simplom organic and one incoganic spacient, including all nonbonding
Chapter 5 Solutions
Organic Chemistry
Ch. 5.2 - Prob. 1PCh. 5.2 - Prob. 2PCh. 5.2 - Prob. 3PCh. 5.2 - Prob. 4PCh. 5.3 - Is cocaine (Worked Example 5-2) dextrorotatory or...Ch. 5.3 - Prob. 6PCh. 5.5 - Prob. 7PCh. 5.5 - Prob. 8PCh. 5.5 - Prob. 9PCh. 5.5 - Assign R or S configuration to the chirality...
Ch. 5.5 - Draw a tetrahedral representation of...Ch. 5.5 - Prob. 12PCh. 5.6 - One of the following molecules (a)–(d) is...Ch. 5.6 - Prob. 14PCh. 5.6 - Assign R or S configuration to each chirality...Ch. 5.7 - Prob. 16PCh. 5.7 - Which of the following have a meso form? (Recall...Ch. 5.7 - Does the following structure represent a meso...Ch. 5.8 - Prob. 19PCh. 5.8 - Prob. 20PCh. 5.9 - Prob. 21PCh. 5.11 - Prob. 22PCh. 5.11 - Prob. 23PCh. 5.11 - The lactic acid that builds up in tired muscles is...Ch. 5.11 - The aconitase-catalyzed addition of water to...Ch. 5.SE - Which of the following structures are identical?...Ch. 5.SE - Prob. 27VCCh. 5.SE - Prob. 28VCCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Prob. 30VCCh. 5.SE - Prob. 31APCh. 5.SE - Which of the following compounds are chiral? Draw...Ch. 5.SE - Prob. 33APCh. 5.SE - Eight alcohols have the formula C5H12O. Draw them....Ch. 5.SE - Draw compounds that fit the following...Ch. 5.SE - Prob. 36APCh. 5.SE - Prob. 37APCh. 5.SE - Prob. 38APCh. 5.SE - What is the stereochemical configuration of the...Ch. 5.SE - Prob. 40APCh. 5.SE - Prob. 41APCh. 5.SE - Prob. 42APCh. 5.SE - Prob. 43APCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Prob. 46APCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Assign R or S configurations to the chirality...Ch. 5.SE - Assign R or S stereochemistry to the chirality...Ch. 5.SE - Prob. 50APCh. 5.SE - Draw examples of the following: (a) A meso...Ch. 5.SE - Prob. 52APCh. 5.SE - Prob. 53APCh. 5.SE - Prob. 54APCh. 5.SE - On reaction with hydrogen gas by a platinum...Ch. 5.SE - Prob. 56APCh. 5.SE - Prob. 57APCh. 5.SE - One of the steps in fat metabolism is the...Ch. 5.SE - The dehydration of citrate to yield cis-aconitate,...Ch. 5.SE - The first step in the metabolism of glycerol,...Ch. 5.SE - One of the steps in fatty-acid biosynthesis is the...Ch. 5.SE - Prob. 62APCh. 5.SE - Draw tetrahedral representations of the two...Ch. 5.SE - The naturally occurring form of the amino acid...Ch. 5.SE - Prob. 65APCh. 5.SE - Prob. 66APCh. 5.SE - Prob. 67APCh. 5.SE - Allenes are compounds with adjacent carbon-carbon...Ch. 5.SE - Prob. 69APCh. 5.SE - Prob. 70APCh. 5.SE - How many stereoisomers of...Ch. 5.SE - Draw both cis- and trans-1,4-dimethylcyclohexane...Ch. 5.SE - Draw both cis- and trans-1,3-dimethylcyclohexane...Ch. 5.SE - cis-1,2-Dimethylcyclohexane is optically inactive...Ch. 5.SE - Prob. 75APCh. 5.SE - Prob. 76APCh. 5.SE - Prob. 77AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please provide the structure for this problem, thank you!arrow_forwardDraw the Fischer projection from the skeletal structure shown below. HO OH OH OH OH H Q Drawing Atoms, Bonds and Rings Charges I ☐ T HO H H OH HO I CH2OH H OH Drag H OH -CH2OH CHO -COOH Undo Reset Remove Donearrow_forwardplease provide the structure for this problem, thank youarrow_forward
- presented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forwardReaction A 0,0arrow_forwardpresented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward
- 6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forwardpresented by Mr L How the coprion. (Il Done in no wraction, dew the starting redential) доarrow_forward
- 8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forwardо но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning