
Concept explainers
Which of the following structures are identical? (Green = Cl.)
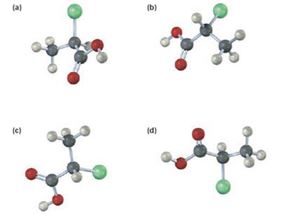
Interpretation:
Among the four structures given which are identical is to be stated.
Concept introduction:
All the four structures given have chiral carbons. By assigning the configuration as R or S the identical structures can be identified.
To state:
Which among the four structures given are identical.
Answer to Problem 26VC
Structures a, b, and d are identical. Structure c is different.
Explanation of Solution
The chiral carbon in all the four structures given is attached to the same four groups, H, Cl, CH3 and COOH but differently oriented. When the sequence rules are applied to these four groups, the order of priority obtained is Cl, COOH, CH3 and H. Using this priorities the configuration of the four structures can be obtained. It is clear that a, b and d have R configuration and d has S configuration. Hence a, b and d represent identical structures and c represents a different configuration.
Structure a, b, and d are identical. Structure c is different.
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry
- Q1: Draw a valid Lewis structures for the following molecules. Include appropriate charges and lone pair electrons. If there is more than one Lewis structure available, draw the best structure. NH3 Sulfate Boron tetrahydride. C3H8 (linear isomer) OCN NO3 CH3CN SO2Cl2 CH3OH2*arrow_forwardIn the following molecule, indicate the hybridization and shape of the indicated atoms. -z: CH3 CH3 H3C HO: CI: :arrow_forwardQ3: Draw the Lewis structures for nitromethane (CH3NO2) and methyl nitrite (CH3ONO). Draw at least two resonance forms for each. Determine which form for each is the major resonance contributor. Page 1 of 4 Chem 0310 Organic Chemistry 1 Recitations Q4: Draw the Lewis structures for the cyanate ion (OCN) and the fulminate ion (CNO-). Draw all possible resonance structures for each. Determine which form for each is the major resonance contributor.arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

