
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5.SE, Problem 70AP
Interpretation Introduction
Interpretation:
The light induced reaction of (S)-1-chloro-2-methylbutane is to be explained.
Concept introduction:
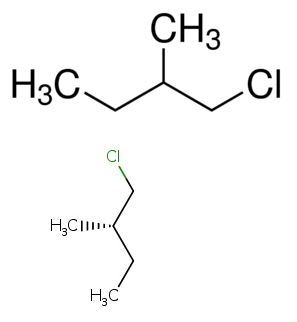
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
2. Write a reasonable mechanism that converts the reactants into the products. Avoid issues A-U
from the previous page. You can use any number of steps (it does not have to be a one-step
mechanism). Do not use any other chemicals (solvents, acids, bases, etc.) in your mechanism.
2
2
H
ΗΘ
For the following reaction, the partial pressures were determined for the reaction components as shownbelow. Is the reaction at equilibrium? If not, in which direction will it proceed?I2 (g) + Cl2 (g) ⇋ 2 ICl (g) Kp = 81.9 partial pressures:
I2 = 0.114 atm; Cl2 = 0.102 atm; ICl = 0.355 atm
Draw the major product of this reaction. Ignore inorganic byproducts.
H3O+
+
O
Chapter 5 Solutions
Organic Chemistry
Ch. 5.2 - Prob. 1PCh. 5.2 - Prob. 2PCh. 5.2 - Prob. 3PCh. 5.2 - Prob. 4PCh. 5.3 - Is cocaine (Worked Example 5-2) dextrorotatory or...Ch. 5.3 - Prob. 6PCh. 5.5 - Prob. 7PCh. 5.5 - Prob. 8PCh. 5.5 - Prob. 9PCh. 5.5 - Assign R or S configuration to the chirality...
Ch. 5.5 - Draw a tetrahedral representation of...Ch. 5.5 - Prob. 12PCh. 5.6 - One of the following molecules (a)–(d) is...Ch. 5.6 - Prob. 14PCh. 5.6 - Assign R or S configuration to each chirality...Ch. 5.7 - Prob. 16PCh. 5.7 - Which of the following have a meso form? (Recall...Ch. 5.7 - Does the following structure represent a meso...Ch. 5.8 - Prob. 19PCh. 5.8 - Prob. 20PCh. 5.9 - Prob. 21PCh. 5.11 - Prob. 22PCh. 5.11 - Prob. 23PCh. 5.11 - The lactic acid that builds up in tired muscles is...Ch. 5.11 - The aconitase-catalyzed addition of water to...Ch. 5.SE - Which of the following structures are identical?...Ch. 5.SE - Prob. 27VCCh. 5.SE - Prob. 28VCCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Prob. 30VCCh. 5.SE - Prob. 31APCh. 5.SE - Which of the following compounds are chiral? Draw...Ch. 5.SE - Prob. 33APCh. 5.SE - Eight alcohols have the formula C5H12O. Draw them....Ch. 5.SE - Draw compounds that fit the following...Ch. 5.SE - Prob. 36APCh. 5.SE - Prob. 37APCh. 5.SE - Prob. 38APCh. 5.SE - What is the stereochemical configuration of the...Ch. 5.SE - Prob. 40APCh. 5.SE - Prob. 41APCh. 5.SE - Prob. 42APCh. 5.SE - Prob. 43APCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Prob. 46APCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Assign R or S configurations to the chirality...Ch. 5.SE - Assign R or S stereochemistry to the chirality...Ch. 5.SE - Prob. 50APCh. 5.SE - Draw examples of the following: (a) A meso...Ch. 5.SE - Prob. 52APCh. 5.SE - Prob. 53APCh. 5.SE - Prob. 54APCh. 5.SE - On reaction with hydrogen gas by a platinum...Ch. 5.SE - Prob. 56APCh. 5.SE - Prob. 57APCh. 5.SE - One of the steps in fat metabolism is the...Ch. 5.SE - The dehydration of citrate to yield cis-aconitate,...Ch. 5.SE - The first step in the metabolism of glycerol,...Ch. 5.SE - One of the steps in fatty-acid biosynthesis is the...Ch. 5.SE - Prob. 62APCh. 5.SE - Draw tetrahedral representations of the two...Ch. 5.SE - The naturally occurring form of the amino acid...Ch. 5.SE - Prob. 65APCh. 5.SE - Prob. 66APCh. 5.SE - Prob. 67APCh. 5.SE - Allenes are compounds with adjacent carbon-carbon...Ch. 5.SE - Prob. 69APCh. 5.SE - Prob. 70APCh. 5.SE - How many stereoisomers of...Ch. 5.SE - Draw both cis- and trans-1,4-dimethylcyclohexane...Ch. 5.SE - Draw both cis- and trans-1,3-dimethylcyclohexane...Ch. 5.SE - cis-1,2-Dimethylcyclohexane is optically inactive...Ch. 5.SE - Prob. 75APCh. 5.SE - Prob. 76APCh. 5.SE - Prob. 77AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the law of mass action for the following quilibrium 2N2O5(g) --> 4 NO2(g) + O2(g).arrow_forwardState the products (formulas) of the reaction of acetophenone with iodine and NaOH.arrow_forwardCh. 4- Precipitation Reactions Worksheet Write balanced, complete ionic, and net ionic equations for the following reactions that mav produce precipitates. Use NR to indicate no reaction Ave 1\ +3 =6 Fe + V-2 Na S04 13. Write the balanced equation for the reaction of iron (III) phosphate with sodium sulfate to make iron (III) sulfate and sodium phosphate. 2FePO4 + M, Soy a) If you perform this reaction with 25 grams of iron (III) phosphate and an excess of sodium sulfate, how many grams of iron (III) sulfate can you make? 21 Fe 2 3x 1 Na 3 25g Fe Ingle 150,829 Indes 2 nol 3 1335 349.89 35.90 Ihol & Sanz Fez Bak heck 3x1 50ab) If 18.5 grams of iron (III) sulfate are actually made when you do this reaction, what is your Poy percent yield? 118.5 259-1-100 51.4% (0.74)x100610 335 If you do this reaction with 15 grams of sodium sulfate and get a 65.0% yield, how many grams of sodium phosphate will you make? 10.59 14. Ammonia is produced from the reaction of nitrogen and hydrogen according…arrow_forward
- == Functional Groups Identifying and drawing hemiacetals and acetals In the drawing area below, create an acetal with 1 isopropoxy group, 1 hydroxyl group, and a total of 10 carbon atoms. Explanation Click and drag to start drawing a structure. Check G +arrow_forwardState the products (formulas) of the reaction of acetophenone with iodine and NaOH.arrow_forwardExplanation Check Draw the skeletal ("line") structure of 5-hydroxy-4-methyl-2-pentanone. Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cer ☐ : Carrow_forward
- 1. Using a Model set Build a model for the following compound [CH2BrCI]. 2. Build another model of the mirror image of your first molecule. 3. Place the two models next to each other and take a picture which shows the differences between the two models. 4. Determine the absolute stereochemistry R or S for the two models. 5. Write or type a paragraph to Discuss the stereochemical relationship between the two models of CH2BrCl. You must provide an explanation for your conclusions also provide a description for the colors used to represent each atom in the model's images.arrow_forwardWhat parameters are included in the specific rotation calculation of a pure substance based on measurement from a polarimeter? Select one or more: Density of the sample Pathlength of the sample container Enantiomeric excess of the sample Measured rotation of lightarrow_forwardV Determine whether the following molecule is a hemiacetal, acetal, or neither and select the appropriate box below. Also, highlight the hemiacetal or acetal carbon if there is one. Explanation O CH O Ohemiacetal Oacetal Oneither Check A 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Cer 000 Ararrow_forward
- 1. Using Online resources and chemical structures hand draw four different organic compounds (not those already shown in your handout) that are chiral, optically active (a pair of enantiomers will count as one). Pay attention to correct stereochemistry 2. Write or type a short paragraph to Discuss the stereochemical relationship between the four compounds.arrow_forward1. Using a Model set Build a model for the following compound [CHBRIF] 2. Build another model of the mirror image of your first molecule. 3. Place the two models next to each other and take a picture which shows the differences between the two models. 4. Determine the absolute stereochemistry R or S for the two models. 5. Write or type a paragraph to Discuss the stereochemical relationship between the two models of CHBгCIF. You must provide an explanation for your conclusions also provide a description for the colors used to representarrow_forwardThe specific rotation of a sample depends upon measured angle of rotation, the density of the sample, and the pathway length of the light. True Falsearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning