
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5.9, Problem 21P
Interpretation Introduction
Interpretation:
The relationship between this pair of isomers is to be determined.
Concept introduction:
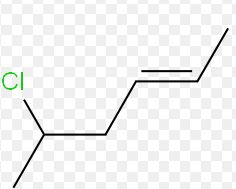
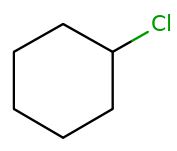
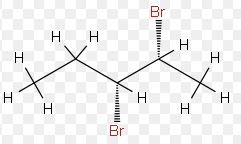
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Choosing reagents and conditions for acetal formation or hydrolysis
0/5
A student proposes the transformation below in one step of an organic synthesis. There may be one or more products missing from
the right-hand side, but there are no reagents missing from the left-hand side. There may also be catalysts, small inorganic
reagents, and other important reaction conditions missing from the arrow.
• Is the student's transformation possible? If not, check the box under the drawing area.
If the student's transformation is possible, then complete the reaction by adding any missing products to the right-hand side,
and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow.
• You do not need to balance the reaction, but be sure every important organic reactant or product is shown.
+
This transformation can't be done in one step.
5
I
H
Autumn
alo
值
Ar
B
A block of copper of mass
2.00kg(cp
= 0.3851
.K) and
g
temperature 0°C is introduced into
an insulated container in which there
is 1.00molH, O(g) at 100°C and 1.00
2
atm. Note that
C
P
= 4.184. K for liquid water, and
g
that A
H = 2260 for water.
vap
g
Assuming all the steam is condensed
to water, and that the pressure
remains constant:
(a) What will be the final temperature
of the system?
(b) What is the heat transferred from
the water to the copper?
(c) What is the entropy change of the
water, the copper, and the total
system?
Identify the missing organic reactants in the following reaction:
H+
X + Y
OH
H+
O
O
Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule
reactants or products (like H₂O) are not shown.
In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the
structures in any arrangement that you like, so long as they aren't touching.
Explanation
Check
Click and drag to start drawing a
structure.
X
G
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cente
?
E
Chapter 5 Solutions
Organic Chemistry
Ch. 5.2 - Prob. 1PCh. 5.2 - Prob. 2PCh. 5.2 - Prob. 3PCh. 5.2 - Prob. 4PCh. 5.3 - Is cocaine (Worked Example 5-2) dextrorotatory or...Ch. 5.3 - Prob. 6PCh. 5.5 - Prob. 7PCh. 5.5 - Prob. 8PCh. 5.5 - Prob. 9PCh. 5.5 - Assign R or S configuration to the chirality...
Ch. 5.5 - Draw a tetrahedral representation of...Ch. 5.5 - Prob. 12PCh. 5.6 - One of the following molecules (a)–(d) is...Ch. 5.6 - Prob. 14PCh. 5.6 - Assign R or S configuration to each chirality...Ch. 5.7 - Prob. 16PCh. 5.7 - Which of the following have a meso form? (Recall...Ch. 5.7 - Does the following structure represent a meso...Ch. 5.8 - Prob. 19PCh. 5.8 - Prob. 20PCh. 5.9 - Prob. 21PCh. 5.11 - Prob. 22PCh. 5.11 - Prob. 23PCh. 5.11 - The lactic acid that builds up in tired muscles is...Ch. 5.11 - The aconitase-catalyzed addition of water to...Ch. 5.SE - Which of the following structures are identical?...Ch. 5.SE - Prob. 27VCCh. 5.SE - Prob. 28VCCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Prob. 30VCCh. 5.SE - Prob. 31APCh. 5.SE - Which of the following compounds are chiral? Draw...Ch. 5.SE - Prob. 33APCh. 5.SE - Eight alcohols have the formula C5H12O. Draw them....Ch. 5.SE - Draw compounds that fit the following...Ch. 5.SE - Prob. 36APCh. 5.SE - Prob. 37APCh. 5.SE - Prob. 38APCh. 5.SE - What is the stereochemical configuration of the...Ch. 5.SE - Prob. 40APCh. 5.SE - Prob. 41APCh. 5.SE - Prob. 42APCh. 5.SE - Prob. 43APCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Prob. 46APCh. 5.SE - Assign R or S configuration to each chirality...Ch. 5.SE - Assign R or S configurations to the chirality...Ch. 5.SE - Assign R or S stereochemistry to the chirality...Ch. 5.SE - Prob. 50APCh. 5.SE - Draw examples of the following: (a) A meso...Ch. 5.SE - Prob. 52APCh. 5.SE - Prob. 53APCh. 5.SE - Prob. 54APCh. 5.SE - On reaction with hydrogen gas by a platinum...Ch. 5.SE - Prob. 56APCh. 5.SE - Prob. 57APCh. 5.SE - One of the steps in fat metabolism is the...Ch. 5.SE - The dehydration of citrate to yield cis-aconitate,...Ch. 5.SE - The first step in the metabolism of glycerol,...Ch. 5.SE - One of the steps in fatty-acid biosynthesis is the...Ch. 5.SE - Prob. 62APCh. 5.SE - Draw tetrahedral representations of the two...Ch. 5.SE - The naturally occurring form of the amino acid...Ch. 5.SE - Prob. 65APCh. 5.SE - Prob. 66APCh. 5.SE - Prob. 67APCh. 5.SE - Allenes are compounds with adjacent carbon-carbon...Ch. 5.SE - Prob. 69APCh. 5.SE - Prob. 70APCh. 5.SE - How many stereoisomers of...Ch. 5.SE - Draw both cis- and trans-1,4-dimethylcyclohexane...Ch. 5.SE - Draw both cis- and trans-1,3-dimethylcyclohexane...Ch. 5.SE - cis-1,2-Dimethylcyclohexane is optically inactive...Ch. 5.SE - Prob. 75APCh. 5.SE - Prob. 76APCh. 5.SE - Prob. 77AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the solubility of CaF2 in g/L (Kp = 4.0 x 10-8). sparrow_forwardFor the following reaction with excess reagent, predict the product. Be sure your answer accounts for stereochemistry. If multiple stereocenters are formed, be sure to draw all products using appropriate wedges and dashes. 1. EtLi, Et₂O CH₁ ? 2. H₂O*arrow_forwardWrite the systematic name of each organic molecule: structure 요 OH ہو۔ HO OH name X S ☐ ☐arrow_forward
- Predict the major products of this organic reaction. If there aren't any products, because nothing will happen, check the box under the drawing area instead. D ㄖˋ ید H No reaction. + 5 H₂O.* Click and drag to start drawing a structure. OH H₂Oarrow_forwardDraw one product of an elimination reaction between the molecules below. Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side products of the reaction 'O 10 + x 也 HO + 义 Click and drag to start drawing a structure.arrow_forwardWhat are the angles a and b in the actual molecule of which this is a Lewis structure? H- :0: C=N: b Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes. a = 0° b=0 Xarrow_forward
- A student proposes the transformation below in one step of an organic synthesis. There may be one or more products missing from the right-hand side, but there are no reagents missing from the left-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. • If the student's transformation is possible, then complete the reaction by adding any missing products to the right-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + This transformation can't be done in one step. T iarrow_forwardDetermine the structures of the missing organic molecules in the following reaction: H+ O OH H+ + H₂O ☑ ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structure of the missing organic molecule X. Molecule X shows up in multiple steps, but you only have to draw its structure once. Click and drag to start drawing a structure. X § ©arrow_forwardTable 1.1 Stock Standard Solutions Preparation. The amounts shown should be dissolved in 100 mL. Millipore water. Calculate the corresponding anion concentrations based on the actual weights of the reagents. Anion Amount of reagent (g) Anion Concentration (mg/L) 0.1649 Reagent Chloride NaCl Fluoride NaF 0.2210 Bromide NaBr 0.1288 Nitrate NaNO3 0.1371 Nitrite NaNO2 0.1500 Phosphate KH2PO4 0.1433 Sulfate K2SO4 0.1814arrow_forward
- Draw the structure of the pound in the provided CO as a 300-1200 37(2), 11 ( 110, and 2.5 (20arrow_forwardPlease help me with # 4 and 5. Thanks in advance!arrow_forwardA small artisanal cheesemaker is testing the acidity of their milk before it coagulates. During fermentation, bacteria produce lactic acid (K₁ = 1.4 x 104), a weak acid that helps to curdle the milk and develop flavor. The cheesemaker has measured that the developing mixture contains lactic acid at an initial concentration of 0.025 M. Your task is to calculate the pH of this mixture and determine whether it meets the required acidity for proper cheese development. To achieve the best flavor, texture and reduce/control microbial growth, the pH range needs to be between pH 4.6 and 5.0. Assumptions: Lactic acid is a monoprotic acid H H :0:0: H-C-C H :0: O-H Figure 1: Lewis Structure for Lactic Acid For simplicity, you can use the generic formula HA to represent the acid You can assume lactic acid dissociation is in water as milk is mostly water. Temperature is 25°C 1. Write the K, expression for the dissociation of lactic acid in the space provided. Do not forget to include state symbols.…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning