
General, Organic, & Biological Chemistry
3rd Edition
ISBN: 9780073511245
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 5.77P
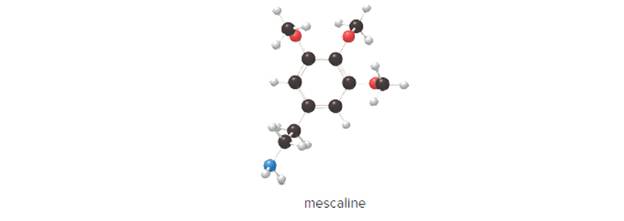
Mescaline is a hallucinogen in peyote, a cactus native to the southwestern UnitedStates and Mexico. (a) What is the chemical formula of mescaline? (b) Calculate itsmolar mass. (c) How many moles are contained in 7.50 g of mescaline?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Provide a complete IUPAC name for each of the following compounds.
a)
b)
c)
OH
OH
OH
a)
b)
c)
2. Provide a complete IUPAC name for each of the following compounds.
a)
b)
a)
OH
b)
он
c)
OB
>=
c)
3. Provide a common name for each of the following alcohols.
a)
a)
OH
b)
OH
c)
HO
b)
c)
4. Provide a common name for each of the following compounds.
b)
OH
a)
5
a)
Y
OH
c)
OH
Using the critical constants for water
(refer to the table in the lecture slides),
calculate the second virial coefficient.
Assume that the compression factor (Z)
is expressed as an expansion series in
terms of pressure.
Chapter 5 Solutions
General, Organic, & Biological Chemistry
Ch. 5.1 - Use the molecular art to identify the process as a...Ch. 5.1 - Use the molecular art to identify the process as a...Ch. 5.1 - Label the reactants and products, and indicate how...Ch. 5.1 - One term in a balanced chemical equation contained...Ch. 5.2 - Write a balanced equation for each reaction....Ch. 5.2 - Write a balanced equation for the following...Ch. 5.2 - Write a balanced equation for the reaction of...Ch. 5.2 - Balance each chemical equation. Al+H2SO4Al2(...Ch. 5.3 - Write a balanced equation for the reaction...Ch. 5.3 - Classify each reaction as a combination or...
Ch. 5.3 - Classify each reaction as a single replacement or...Ch. 5.3 - Classify each reaction as a combination,...Ch. 5.3 - Fill in the needed reactants or products for each...Ch. 5.4 - Identify the species that is oxidized and the...Ch. 5.4 - Identify the species that is oxidized and the...Ch. 5.4 - Prob. 5.16PCh. 5.5 - How many items are contained in one mole of (a)...Ch. 5.5 - How many carbon atoms are contained in each of the...Ch. 5.5 - How many molecules are contained in each of the...Ch. 5.5 - How many moles of water contain each of the...Ch. 5.6 - Calculate the formula weight of each ionic...Ch. 5.6 - The unmistakable odor of a freshly cut cucumber is...Ch. 5.6 - Prob. 5.23PCh. 5.6 - Calculate the number of grams contained in each of...Ch. 5.6 - How many moles are contained in each of the...Ch. 5.6 - How many molecules are contained in two 500.-mg...Ch. 5.7 - Use the balanced equation for the reaction of N2...Ch. 5.7 - Use the balanced equation in Sample Problem 5.15...Ch. 5.8 - Prob. 5.29PCh. 5.8 - Using the balanced equation for the combustion of...Ch. 5.8 - Prob. 5.31PCh. 5.8 - Use the balanced equation, N2+O22NO, to answer the...Ch. 5.9 - Prob. 5.33PCh. 5.9 - Prob. 5.34PCh. 5.9 - The synthetic antiviral drug Tamiflu, currently...Ch. 5.10 - Consider the reaction of hydrogen and nitrogen to...Ch. 5.10 - Using the balanced equation for the reaction of H2...Ch. 5.10 - Using the balanced equation, 3H2(g)+N2(g)2NH3(g),...Ch. 5.10 - Using the balanced equation, N2(g)+O2(g)2NO(g),...Ch. 5.10 - Prob. 5.41PCh. 5.10 - Prob. 5.42PCh. 5 - Prob. 5.43PCh. 5 - Prob. 5.44PCh. 5 - How many atoms of each element are drawn on each...Ch. 5 - How many atoms of each element are drawn on each...Ch. 5 - Use the molecular art to write a balanced equation...Ch. 5 - Prob. 5.48PCh. 5 - Balance each equation. a....Ch. 5 - Balance each equation. a....Ch. 5 - Prob. 5.51PCh. 5 - Prob. 5.52PCh. 5 - Prob. 5.53PCh. 5 - Prob. 5.54PCh. 5 - For the reaction depicted in the molecular art:...Ch. 5 - Prob. 5.56PCh. 5 - Prob. 5.57PCh. 5 - Prob. 5.58PCh. 5 - Classify the reaction depicted in the molecular...Ch. 5 - Classify the reaction depicted in the molecular...Ch. 5 - Classify each reaction as combination,...Ch. 5 - Classify each reaction as combination,...Ch. 5 - Fill in the needed reactant or product for each of...Ch. 5 - Fill in the needed reactant or product for each of...Ch. 5 - Identify the species that is oxidized and the...Ch. 5 - Identify the species that is oxidized and the...Ch. 5 - Prob. 5.67PCh. 5 - Rechargeablenickel-cadmium batteries are used in...Ch. 5 - The reaction of hydrogen (H2) with acetylene...Ch. 5 - Prob. 5.70PCh. 5 - Calculate the formula weight and molar mass of...Ch. 5 - Calculate the formula weight and molar mass of...Ch. 5 - L-Dopa is a drug used to treat Parkinson’s...Ch. 5 - Niacin, vitamin B3, is found in soybeans, which...Ch. 5 - Which quantity has the greater mass? 1 mol of Fe...Ch. 5 - Prob. 5.76PCh. 5 - Mescaline is a hallucinogen in peyote, a cactus...Ch. 5 - Prob. 5.78PCh. 5 - How many grams are contained in 5.00 mol of each...Ch. 5 - How many grams are contained in 0.50 mol of each...Ch. 5 - Prob. 5.81PCh. 5 - How many moles are contained in each number of...Ch. 5 - Prob. 5.83PCh. 5 - Prob. 5.84PCh. 5 - Prob. 5.85PCh. 5 - Prob. 5.86PCh. 5 - Using the balanced equation for the combustion of...Ch. 5 - Sodium metal (Na) reacts violently when added to...Ch. 5 - Prob. 5.89PCh. 5 - Prob. 5.90PCh. 5 - What is the percent yield of B in a reaction that...Ch. 5 - What is the percent yield of B in a reaction that...Ch. 5 - The reaction of methane (CH4) with Cl2forms...Ch. 5 - Methanol (CH4O), which is used as a fuel in...Ch. 5 - Consider the given reaction mixture that contains...Ch. 5 - Consider the reaction of A2 and B2 to form A2B,...Ch. 5 - Prob. 5.97PCh. 5 - Prob. 5.98PCh. 5 - Prob. 5.99PCh. 5 - Prob. 5.100PCh. 5 - The local anesthetic ethyl chloride ( C2H5Cl,...Ch. 5 - The solvent dichloromethane (, molar mass 84.93...Ch. 5 - Answer the following questions about the...Ch. 5 - Answer the following questions about diethyl ether...Ch. 5 - Prob. 5.105PCh. 5 - Prob. 5.106PCh. 5 - Prob. 5.107PCh. 5 - Prob. 5.108PCh. 5 - Prob. 5.109PCh. 5 - Prob. 5.110PCh. 5 - DDT, a pesticide that kills disease-carrying...Ch. 5 - Prob. 5.112PCh. 5 - TCDD, also called dioxin...Ch. 5 - Prob. 5.114CP
Additional Science Textbook Solutions
Find more solutions based on key concepts
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Give the IUPAC name for each compound.
Organic Chemistry
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- +3413 pts /4800 Question 38 of 48 > Write the full electron configuration for a Kion. © Macmillan Learning electron configuration: ↓ Resources Solution Penalized → Al Tutor Write the full electron configuration for an Fion. electron configuration: T G 6 & 7 Y H כ Y 00 8 hp 9 J K no L 144 P 112 | t KC 47°F Clear ins prt sc delete ] backspace erarrow_forwardHow to solve these types of problems step by step? I'm so confused.arrow_forwardIdentify the expected product of the following Claisen rearrangement. || = IV OV 00000 5 ОН Он Он Он Он || III IV Varrow_forward
- Can you please color-code and explain how to solve this and any molecular orbital diagram given? I'm so confused; could you provide baby steps regardless of which problem type they gave me?arrow_forwardConsider the following structure. OH Esmolol The synthesis of this compound uses a building block derived from either ethylene oxide or epichlorohydrin. 1) Determine which building block was used: | 2) Draw the structure of the nucleophiles that were used along with this building block in the synthesis of the molecule. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. You do not have to consider stereochemistry. Θε {n [arrow_forward< 10:44 5GW 10 Question 7/8 Show Answer Convert 46.0 mm to inches (1 inch = 2.54 cm) 46.0 DAM STARTING AMOUNT 1 cm 1 in 46.0 mm x ☑ 10 mm 10 cm ADD FACTOR DELETE x() X × = 1.81 in = 1 10 Dam ANSWER RESET ១ 2.54 0.0460 mm 10 1000 in 0.001 11.7 m 4.60 18.1 cm 100 1.81 0.394 1 0.1 46.0 0.01 Tap here for additional resourcesarrow_forward
- < 10:44 Question 6/8 5GW (10 Submit A cake recipe calls for 230.0 mL of buttermilk. How 230.0 many cups is this? DAL STARTING AMOUNT × 1 cups 230.0 mL x = 0.9722 cups 230.0 mL ADD FACTOR DELETE (( ) = 1 cups 230.0 DAE ANSWER RESET ១ 9.722 × 105 0.8706 cups 8.706 × 104 1 L 8.706 × 105 0.9722 quart 10 100 mL 0.001 0.1 6.076 × 103 0.01 9.722 × 104 230.0 0.06076 4 1.0567 1000 6.076 × 104 Tap here for additional resourcesarrow_forward< 10:44 Question 6/8 5GW (10 Submit A cake recipe calls for 230.0 mL of buttermilk. How 230.0 many cups is this? DAL STARTING AMOUNT × 1 cups 230.0 mL x = 0.9722 cups 230.0 mL ADD FACTOR DELETE (( ) = 1 cups 230.0 DAE ANSWER RESET ១ 9.722 × 105 0.8706 cups 8.706 × 104 1 L 8.706 × 105 0.9722 quart 10 100 mL 0.001 0.1 6.076 × 103 0.01 9.722 × 104 230.0 0.06076 4 1.0567 1000 6.076 × 104 Tap here for additional resourcesarrow_forwardShow work in detailed of all the options. Don't give Ai generated solutionarrow_forward
- Predict the Product. Predict the major organic product for the following reaction:arrow_forwardPlease provide the complete mechanism for the reaction below including arrows, intermediates, and formal charges.arrow_forwardCan you please explain this to me? Maybe color-code it in essence and highlight it.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Step by Step Stoichiometry Practice Problems | How to Pass ChemistryMole Conversions Made Easy: How to Convert Between Grams and Moles; Author: Ketzbook;https://www.youtube.com/watch?v=b2raanVWU6c;License: Standard YouTube License, CC-BY