
Methanol is synthesized from carbon monoxide and hydrogen in the reaction
A process ?owchart is shown below.
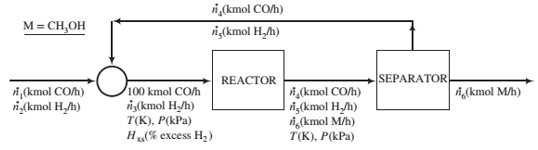
The fresh feed to the system, which contains only CO and H2, is blended with a recycle stream containing the same species. The combined stream is heated and compressed to a temperature T(K) and a pressure P(kPa) and fed to the reactor. The percentage excess hydrogen in this stream is Hxs. The reactor ef?uent—also at T and P—goes to a separation unit where essentially all of the methanol produced in the reactor is condensed and removed as product. The unreacted CO and H2constitute the recycle stream blended with the fresh feed.
Provided that the reaction temperature (and hence the rate of reaction) is high enough and the ideal- gas equation of state is a reasonable approximation at the reactor outlet conditions (a questionable assumption), the ratio
In these equations, piis the partial pressure of species i in kilopascals
Suppose P = 5000 kPa, T = 500 K, and the percentage excess of hydrogen in the feed to the reactor (Hxs) = 5.0%. Calculate
- ) and the ?ow rate (SCMH) of the recycle stream.
Run the program for the following nine conditions (three of which are the same):
(c) You should ?nd that the methanol yield increases with increasing pressure and decreasing temperature. What cost is associated with increasing the pressure?
(d) Why might the yield be much lower than the calculated value if the temperature is too low?
(e) If you actually ran the reaction at the given conditions and analyzed the reactor ef?uent, why might the spreadsheet values in Columns F—M be signi?cantly different from the measured values of these quantities? (Give several reasons, including assumptions made in obtaining the spreadsheet values.)
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Elementary Principles of Chemical Processes, Binder Ready Version
Additional Engineering Textbook Solutions
Modern Database Management
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
Degarmo's Materials And Processes In Manufacturing
Starting Out with C++ from Control Structures to Objects (9th Edition)
INTERNATIONAL EDITION---Engineering Mechanics: Statics, 14th edition (SI unit)
Concepts Of Programming Languages
- A process for the microbial synthesis of 1,3-propanediol ( 3 8 2 C H O ) uses an anaerobicfermenter with a selected strain of K. pneumoniae to convert glycerol ( 3 8 3 C H O ) to 1,3-propanediol and acetic acid ( 2 4 2 C H O ). All other byproducts are of negligible concentration.The fermentation and cell growth equation can be written:3 8 3 3 4 7 2 3 8 2 2 4 2 2 2 68 3 3 49 15 15 40 C H O NH C H O N C H O C H O CO H O + → + + + +The continuous fermentation process is set up at 37°C and atmospheric pressure.Anaerobic conditions are maintained by sparging the fermentation broth with N2 at aflowrate of 500 litres per minute. The medium, containing ammonia, is fed at 500 kg perhour, and has a composition of 14% (w/w) glycerol. Suppression of the side reactions isachieved by excess glycerol, so the liquid product contains 3% (w/w) unreacted glycerol.2a. Draw a process diagram. List all your assumptions necessary to write a materialbalance.(5 marks)2b. List your unknowns.(3 marks)2c. Write…arrow_forward8-4. A pressurized-water reactor generates 70 Mw(t) in the core. The coolant-moderator mass-flow rate is 107 lbm/hr. It enters the core at 490°F. Estimate the effective thermal- neutron fission cross section in the core.arrow_forwardQ/ 8-17 cylindrical reactor core is 4 ft in diameter and 4.8 ft height. The maximum neutron flux is 1013. The extrapolation length are 0.186 ft in the radial direction and 0.3 ft in the axial direction. The fuel is 20% enriched UO2.0= 500 b. Determine (a) The neutron flux at the upper and lower rims, and (b) the maximum heat generated in the fuel in [MeV/s cm³] and [Btu/hr ft³).arrow_forward
- LATIHAN 8.5-4. Concentration of NaOH Solution in Triple-Effect Evaporator. A forced-circulation triple-effect evaporator using forward feed is to be used to concentrate a 10 wt % NaOH solution entering at 37.8°C to 50%. The steam used enters at 58.6 kPa gage. The absolute pressure in the vapor space of the third effect is 6.76 kPa. The feed rate is 13 608 kg/h. The heat-transfer coefficients are U₁ = 6246, U2 = 3407, and U3 = 2271 W/m² K. All effects have the same area. Calculate the surface area and steam consumption. 8.5-1. Boiling Points in a Triple-Effect Evaporator. A solution with a negligible boiling- point rise is being evaporated in a triple-effect evaporator using saturated steam at 121.1°C (394.3 K). The pressure in the vapor of the last effect is 25.6 kPa abs. The heat-transfer coefficients are U₁ = 2840, U₂ = 1988, and U₁ = 1420 W/m² K and the areas are equal. Estimate the boiling point in each of the evaporators.arrow_forwardThe power generation unit in a plant uses a hot exhaust gas from another process to produce work. The gas enters at 10 bar and 350°C and exits at 1 bar and 40°C. The process produces a net amount of work equal to 4500 J/mol and it exchanges an unknown amount of heat with the surroundings. 1.1 Determine the amount of heat exchanged with the surroundings. Is this heat absorbed or rejected by the system? 1.2 Calculate the entropy change of the exhaust gas. 1.3 As a young and ambitious chemical engineer, you seek ways to improve the process. What is the maximum amount of work that you could extract from this system? Assume that the inlet and outlet conditions of the exhaust gas remain the same. Additional data: Assume the surroundings to be at the constant temperature of 298 K and the exhaust gas to be ideal with CP = 29.3 J/mol.Karrow_forwardLatihan mandiri Reaktor fluidisasi menggunakan katalis padat dengan diameter partikel 0,25 mm, rapat massa 1,50 g/ml, sperisitas 0,90. Pada kondisi unggun diam, porositas 0,35, tinggi unggun 2 m. Gas masuk dari bagian bawah reaktor pada suhu 600°C pada viskositas 0,025 CP serta rapat massa 0,22 lb/cuft. Pada fluidisasi minimum, porositas tercapai pada 0,45. Hitung Hitung a. Laju alir semu minimum (VM) gas masuk kolom fluidisasi ! b. Tinggi unggun jika Vo = 2 VM c. Pressure drop pada kondisi Vo = 2,5 VM < 1 m = 3,28084 ft 1 g/ml = 62,43 lbm/ft³ 1 cp gc = 6,7197 × 10-4 lbm/ft.s = 32,174 ft/s² =arrow_forward
- determine the binary diffusion coefficient of CO2 in air at a) 200 K and 1 atm b)400K and 0.5atm c)600 K and 5 atmarrow_forwardUsing Rachford-Rice in Excel, analyze flash distillation of the following feed stream at P = 1000 kPa and T = 30°C. Feed (1000 kmol/hr) is composed of ethane (25%), propane (30%), propylene (5%) and n-hexane (40%):a. What is the composition and flowrate of the vapor stream? [V should be 196 kmol/hr when solved]b. What is the composition and flowrate of the liquid stream?c. What fraction of the n-hexane (feed) ends up in the vapor phase?d. What fraction of the ethane (feed) ends up in the liquid phase?arrow_forwardA 40 mol % ethanol 60 mol % water mixture at 60 °C and 1 atm is heated. Using Figure 2-3 answer the following:a. At what temperature does the mixture first begin to boil? What is the composition of the first bubble of vapor?b. At what temperature would it stop boiling (assume no material is removed)? What is the composition of the last droplet of liquid?c. At 82 °C, what fraction is liquid? [should be 0.6]d. When 90% has been vaporized, what is the temperature, and what are the liquid and vapor compositions?arrow_forward
- Using Rachford-Rice in Excel, analyze flash distillation of the following feed stream at P = 1000 kPa and T = 30°C. Feed (1000 kmol/hr) is composed of ethane (25%), propane (30%), propylene (5%) and n-hexane (40%):a. What is the composition and flowrate of the vapor stream? [196 kmol/hr]b. What is the composition and flowrate of the liquid stream?c. What fraction of the n-hexane (feed) ends up in the vapor phase?d. What fraction of the ethane (feed) ends up in the liquid phase?arrow_forwardConsidering the molar flux as estimated by the Whitman two-film theory, show the relationship between the mass transfer coefficients based on concentration, and mol fraction gradients, kc and ky, respectively, is given by: ky = Ckc, where C is the total concentration. do not use chatgpt please, i did not understan from it thats why i paid for bartlebyarrow_forwardConsidering the molar flux as estimated by the Whitman two-film theory, show the relationship between the mass transfer coefficients based on concentration, and mol fraction gradients, kc and ky, respectively, is given by: ky = Ckc, where C is the total concentration. please do not use chatgpt, i did not understand from it that is why i paid for this.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





