
Bread is typically made by ?rst dissolving preserved yeast (a microscopic biological organism that consumes sugars and emits CO2 as a waste product) in water, then adding other ingredients, including ?our, sugar, fat (usually butter or shortening), and salt. After the ingredients are combined, the dough is "kneaded," or mixed to promote the formation of a protein network from two proteins (gliadin and glutenin)15 present in wheat ?our. This network is what strengthens the dough and allows it to stretch elastically without breaking. The dough is then allowed to rise in a process called "proo?ng," in which the yeast consumes sugar and releases CO2, which in?ates air pockets in the dough that are subsequently ?lled with air. Finally the dough is baked; the gas pockets expand due to the temperature rise and evaporation of water, the starches from the ?our are dehydrated (dried), and the yeast dies.
A good French bread has an open, porous structure. The pores must be stabilized by the protein network until the bread is dried suf?ciently to hold its shape. The bread collapses if the protein network fails prematurely.
(a) Rouille et al.16 investigated the in?uence of ingredients and mixing conditions on the quality of frozen French bread dough. Each loaf was initially formed roughly as a cylinder with a mass of 150 g (including essentially no CO2), a diameter of 2.0 cm. and a length of 25.0cm. Determine the speci?c volume of a bread dough proofed for two hours at 28°C from which 1.20 cm3 gas/min per 100 g dough evolves as bubbles within the dough. State your assumptions.
(b) During proo?ng, the increases in volume of a series of control loaves were monitored along with the mass of CO2 evolved. Rupture of the protein network during proo?ng can be detected when the volume of the dough no longer increases at the same rate as the production of CO2 from the yeast. Data from one of these experiments are shown in the table below. Plot the speci?c volumes of CO2 (per 100g dough) and dough as a function of time. If the preferred proo?ng time is such that the dough achieves 70% of its total volume before collapse, specify the proper proo?ng time for this formula.
(c) The referenced study found that the parameter with the most signi?cant in?uence on dough quality was mixing time, with an extended mixing time producing a stronger protein network. Why might extended mixing times not be desirable in commercial production of bread?
(d) Suggest causes for the following undesirable bread-baking outcomes: (i) a ?at, dense loaf; (ii) an overly large loaf.
(e) Suggest why the period during which the dough rises is called "proo?ng." Remember that yeast is a biological organism.
| t(min) | 0 | 20 | 40 | 60 | 80 | 100 | 120 | 140 | 160 | 180 | 200 | 220 | 240 |
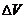 cm3 dough) cm3 dough) |
0 | 0 | 20 | 60 | 80 | 115 | 155 | 198 | 247 | 305 | 322 | 334 | 336 |
| Gas evolved (g CO2) | 0.0 | 37.2 | 63.2 | 68.8 | 126.3 | 192.7 | 234.8 | 315.8 | 385.4 | 515.0 | 578.1 | 657.5 | 745.0 |
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Elementary Principles of Chemical Processes, Binder Ready Version
Additional Engineering Textbook Solutions
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Electric Circuits. (11th Edition)
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
Modern Database Management
Management Information Systems: Managing The Digital Firm (16th Edition)
- At a Pressure of 600 mm Hg, match the substance with the boiling temperature. 54.69°C 1. n-Pentane 49.34°C 2. n-Hexane 3. Acetone 29.32°C く 61.40°C 4. Chloroformarrow_forwardA mixture of oil and gas flows through a horizontal pipe with an inside diameter of 150 mm. The respective volumetric flow rates for the oil and gas are 0.015 and 0.29 m³s-1. Determine the gas void frac- tion and the average velocities of the oil and gas. The friction factor may be assumed to be 0.0045. The gas has a density of 2.4 kgm³ and viscosity of 1 x 10-5 Nsm-2. The oil has a density of 810 kgm³ and density of 0.82 Nsm². Answer: 0.79, 20.8 ms-1, 4 ms-1arrow_forward4. An experimental test rig is used to examine two-phase flow regimes in horizontal pipelines. A particular experiment involved uses air and water at a temperature of 25°C, which flow through a horizontal glass tube with an internal diameter of 25.4 mm and a length of 40 m. Water is admitted at a controlled rate of 0.026 kgs at one end and air at a rate of 5 x 104 kgs in the same direction. The density of water is 1000 kgm³, and the density of air is 1.2 kgm3. Determine the mass flow rate, the mean density, gas void fraction, and the superficial velocities of the air and water. Answer: 0.02605 kgs 1, 61.1 kgm³, 0.94, 0.822 ms-1, 0.051 ms-1arrow_forward
- 1. Determine the range of mean density of a mixture of air in a 50:50 oil-water liquid phase across a range of gas void fractions. The den- sity of oil is 900 kgm³, water is 1000 kgm³, and gas is 10 kgm³. 2. Describe, with the use of sketches, the various flow regimes that can exist in a vertical pipe carrying two-phase flow (liquid and gas).arrow_forwardA mixture of high pressure water and steam at a rate of 0.5 kgs-¹ flows up a vertical tube with an inside diameter of 25.4 mm at a pres- sure 22 bar. Determine the type of flow if the mass quality is 1%. The density of the water is 845 kgm³, the density of steam is 10.8 kgm³, and the viscosity of the water is 1.24 x 104 Nsm2. Answer: Slug flowarrow_forward5. Describe, with the use of sketches, the various two-phase flow regimes that can exist in a horizontal pipe carrying a liquid and a gas. 6. Explain what is meant by gas hold-up and describe ways in which it can be measured.arrow_forward
- A mixture of air and water at a temperature of 25°C flows up through a vertical tube with a length of 4 m and an internal diameter of 25.4 mm with the exit of the tube being at atmospheric pressure. The mass flows of the air and the water are 0.007 kgs¹ and 0.3 kgs-¹, respectively. For air, the density is 1.2 kgm3 and viscosity is 1.85 x 10-5 Nsm-2, and for water, the density is 1000 kgm-3 and viscosity is 8.9 × 10-4 Nsm 2. Answer: 2.7 kNm 2marrow_forwardAt a Pressure of 200 mm Hg, match the substance with the boiling temperature. 69.50°C 1. Benzene 1.92°C 2. Toluene 41.94°C 3. n-Pentane 4. n-Hexane 31.61°Carrow_forwardAt a Pressure of 400 mm Hg, match the substance with the boiling temperature. 62.89°C 1. Styrene 122.69°C 2. Ethanol 3. Toluene 89.48°C 4. Benzene 60.61°Carrow_forward
- 8. A gas is admitted at a rate of 0.015 m³s-¹ to a vertical glass pipe with an inside diameter of 50 mm. The gas bubbles that form travel with a velocity of 32 ms-¹. Determine the gas void fraction and the velocity of the liquid if the volumetric flow is 2.5 x 10-5 m³s-1. Answer: 0.24, 1.7 ms-1 9 Characterise the main concepts of a homogeneous flow model sepa-arrow_forward3. A mixture of air and water at a temperature of 25°C flows up through a vertical tube with a length of 4 m and an internal diameter of 25.4 mm with the exit of the tube being at atmospheric pressure. The mass flows of the air and the water are 0.007 kgs-1 and 0.3 kgs-1, respectively. For air, the density is 1.2 kgm³ and viscosity is 1.85 x 10-5 Nsm-2, and for water, the density is 1000 kgm-3 and viscosity is 8.9 × 10-4 Nsm-2. Answer: 2.7 kNm-2m-1arrow_forward15. Show that for a one-dimensional annular flow in a horizontal pipe with no acceleration, the pressure gradient on the gas core is dp= 4ti dz d√√α where t, is the interfacial shear stress and a is the gas void fraction.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





