
5.50.19 In chemical vapor deposition (CVD), a semiconducting or insulating solid material is formed in a reaction between a gaseous species and a species adsorbed on the surface of silicon wafers (disks about 10 cm in diameter and l mm thick). The coated wafers are subjected to further processing to produce the microelectronic chips in computers and most other electronic devices in use today.
In one such process, silicon dioxide (MW = 60.06, SG = 2.67) is formed in the reaction between gaseous dichlorosilane (DCS) and adsorbed nitrous oxide:
A mixture of DCS and N2O ?ows through a “boat reactor"—a horizontal pipe in which 50 to 100 silicon wafers about 12 cm in diameter and 1 mm thick are set upright along the reactor length, with about 20 mm separation between each wafer. A side view of the reactor is shown below:
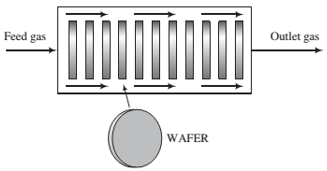
The feed gas enters the reactor at a rate of 3.74 SCMM (standard cubic meters per minute) and contains 22.0 mole% DCS and the balance N2O. In the reactor, the gas ?ows around the wafers, DCS and N2O diffuse into the spaces between the wafers, N2O is adsorbed on the wafer surfaces, and the adsorbed N2O reacts with gaseous DCS. The silicon dioxide formed remains on the surface, and the nitrogen and hydrogen chloride go into the gas phase and eventually leave the reactor with the unconsumed reactants. The temperature and absolute pressure in the reactor are constant at 900°C and 604 millitorr.
(a) The percentage conversion of DCS at a certain axial position (distance along the length of the reactor) is 60%. Calculate the volumetric ?ow rate (m3/min) of gas at this axial position.
(b) The rate of deposition of silicon dioxide per unit area of wafer surface is given by the formula
where
(c) Consider a wafer located at the axial position determined in Part (b). How thick is the silicon dioxide layer on that wafer after two hours of reactor operation, assuming that gas diffusion is rapid enough at the low reactor pressure for the composition of the gas (and hence the component partial pressures) to be uniform over the wafer surface? Express your answer in angstroms, where
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Elementary Principles of Chemical Processes, Binder Ready Version
Additional Engineering Textbook Solutions
Java: An Introduction to Problem Solving and Programming (8th Edition)
Electric Circuits. (11th Edition)
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Management Information Systems: Managing The Digital Firm (16th Edition)
Modern Database Management
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
- A process for the microbial synthesis of 1,3-propanediol ( 3 8 2 C H O ) uses an anaerobicfermenter with a selected strain of K. pneumoniae to convert glycerol ( 3 8 3 C H O ) to 1,3-propanediol and acetic acid ( 2 4 2 C H O ). All other byproducts are of negligible concentration.The fermentation and cell growth equation can be written:3 8 3 3 4 7 2 3 8 2 2 4 2 2 2 68 3 3 49 15 15 40 C H O NH C H O N C H O C H O CO H O + → + + + +The continuous fermentation process is set up at 37°C and atmospheric pressure.Anaerobic conditions are maintained by sparging the fermentation broth with N2 at aflowrate of 500 litres per minute. The medium, containing ammonia, is fed at 500 kg perhour, and has a composition of 14% (w/w) glycerol. Suppression of the side reactions isachieved by excess glycerol, so the liquid product contains 3% (w/w) unreacted glycerol.2a. Draw a process diagram. List all your assumptions necessary to write a materialbalance.(5 marks)2b. List your unknowns.(3 marks)2c. Write…arrow_forward8-4. A pressurized-water reactor generates 70 Mw(t) in the core. The coolant-moderator mass-flow rate is 107 lbm/hr. It enters the core at 490°F. Estimate the effective thermal- neutron fission cross section in the core.arrow_forwardQ/ 8-17 cylindrical reactor core is 4 ft in diameter and 4.8 ft height. The maximum neutron flux is 1013. The extrapolation length are 0.186 ft in the radial direction and 0.3 ft in the axial direction. The fuel is 20% enriched UO2.0= 500 b. Determine (a) The neutron flux at the upper and lower rims, and (b) the maximum heat generated in the fuel in [MeV/s cm³] and [Btu/hr ft³).arrow_forward
- LATIHAN 8.5-4. Concentration of NaOH Solution in Triple-Effect Evaporator. A forced-circulation triple-effect evaporator using forward feed is to be used to concentrate a 10 wt % NaOH solution entering at 37.8°C to 50%. The steam used enters at 58.6 kPa gage. The absolute pressure in the vapor space of the third effect is 6.76 kPa. The feed rate is 13 608 kg/h. The heat-transfer coefficients are U₁ = 6246, U2 = 3407, and U3 = 2271 W/m² K. All effects have the same area. Calculate the surface area and steam consumption. 8.5-1. Boiling Points in a Triple-Effect Evaporator. A solution with a negligible boiling- point rise is being evaporated in a triple-effect evaporator using saturated steam at 121.1°C (394.3 K). The pressure in the vapor of the last effect is 25.6 kPa abs. The heat-transfer coefficients are U₁ = 2840, U₂ = 1988, and U₁ = 1420 W/m² K and the areas are equal. Estimate the boiling point in each of the evaporators.arrow_forwardThe power generation unit in a plant uses a hot exhaust gas from another process to produce work. The gas enters at 10 bar and 350°C and exits at 1 bar and 40°C. The process produces a net amount of work equal to 4500 J/mol and it exchanges an unknown amount of heat with the surroundings. 1.1 Determine the amount of heat exchanged with the surroundings. Is this heat absorbed or rejected by the system? 1.2 Calculate the entropy change of the exhaust gas. 1.3 As a young and ambitious chemical engineer, you seek ways to improve the process. What is the maximum amount of work that you could extract from this system? Assume that the inlet and outlet conditions of the exhaust gas remain the same. Additional data: Assume the surroundings to be at the constant temperature of 298 K and the exhaust gas to be ideal with CP = 29.3 J/mol.Karrow_forwardLatihan mandiri Reaktor fluidisasi menggunakan katalis padat dengan diameter partikel 0,25 mm, rapat massa 1,50 g/ml, sperisitas 0,90. Pada kondisi unggun diam, porositas 0,35, tinggi unggun 2 m. Gas masuk dari bagian bawah reaktor pada suhu 600°C pada viskositas 0,025 CP serta rapat massa 0,22 lb/cuft. Pada fluidisasi minimum, porositas tercapai pada 0,45. Hitung Hitung a. Laju alir semu minimum (VM) gas masuk kolom fluidisasi ! b. Tinggi unggun jika Vo = 2 VM c. Pressure drop pada kondisi Vo = 2,5 VM < 1 m = 3,28084 ft 1 g/ml = 62,43 lbm/ft³ 1 cp gc = 6,7197 × 10-4 lbm/ft.s = 32,174 ft/s² =arrow_forward
- determine the binary diffusion coefficient of CO2 in air at a) 200 K and 1 atm b)400K and 0.5atm c)600 K and 5 atmarrow_forwardUsing Rachford-Rice in Excel, analyze flash distillation of the following feed stream at P = 1000 kPa and T = 30°C. Feed (1000 kmol/hr) is composed of ethane (25%), propane (30%), propylene (5%) and n-hexane (40%):a. What is the composition and flowrate of the vapor stream? [V should be 196 kmol/hr when solved]b. What is the composition and flowrate of the liquid stream?c. What fraction of the n-hexane (feed) ends up in the vapor phase?d. What fraction of the ethane (feed) ends up in the liquid phase?arrow_forwardA 40 mol % ethanol 60 mol % water mixture at 60 °C and 1 atm is heated. Using Figure 2-3 answer the following:a. At what temperature does the mixture first begin to boil? What is the composition of the first bubble of vapor?b. At what temperature would it stop boiling (assume no material is removed)? What is the composition of the last droplet of liquid?c. At 82 °C, what fraction is liquid? [should be 0.6]d. When 90% has been vaporized, what is the temperature, and what are the liquid and vapor compositions?arrow_forward
- Using Rachford-Rice in Excel, analyze flash distillation of the following feed stream at P = 1000 kPa and T = 30°C. Feed (1000 kmol/hr) is composed of ethane (25%), propane (30%), propylene (5%) and n-hexane (40%):a. What is the composition and flowrate of the vapor stream? [196 kmol/hr]b. What is the composition and flowrate of the liquid stream?c. What fraction of the n-hexane (feed) ends up in the vapor phase?d. What fraction of the ethane (feed) ends up in the liquid phase?arrow_forwardConsidering the molar flux as estimated by the Whitman two-film theory, show the relationship between the mass transfer coefficients based on concentration, and mol fraction gradients, kc and ky, respectively, is given by: ky = Ckc, where C is the total concentration. do not use chatgpt please, i did not understan from it thats why i paid for bartlebyarrow_forwardConsidering the molar flux as estimated by the Whitman two-film theory, show the relationship between the mass transfer coefficients based on concentration, and mol fraction gradients, kc and ky, respectively, is given by: ky = Ckc, where C is the total concentration. please do not use chatgpt, i did not understand from it that is why i paid for this.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





