
Concept explainers
The graph here represents the distribution of molecular speeds of hydrogen and neon at 200 K.
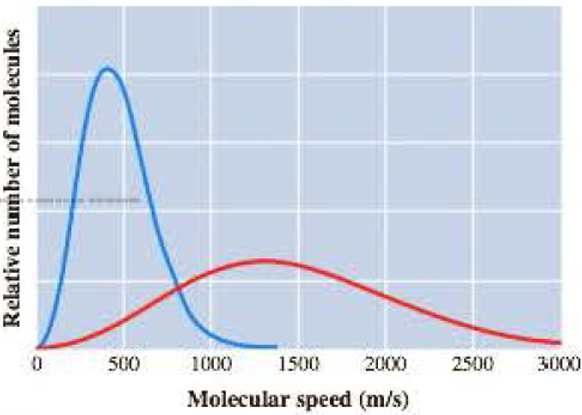
- a Match each curve to the appropriate gas.
- b Calculate the rms speed (in m/s) for each of the gases at 200 K.
- c Which of the gases would you expect to have the greater effusion rate at 200 K? Justify your answer.
- d Calculate the temperature at which the rms speed of the hydrogen gas would equal the rms speed of the neon at 200 K.
(a)
Interpretation:
The curve in the given graph must be matched with the appropriate gas.
Answer to Problem 5.136QP
The taller and narrow curve represents neon atoms
The flatter and wider curve represents hydrogen molecules
Explanation of Solution
Given,
The given graph represents the distribution of molecular speeds of hydrogen and neon at
Matching of given curves with appropriate gases:
In the given graph, the taller and narrow curve whose maximum falls near
The flatter and wider curve whose maximum falls near
The taller and narrow curve matches with neon atoms
The flatter and wider curve matches with hydrogen molecules
(b)
Interpretation:
The rms speed (in
Concept Introduction:
Root-mean-square (rms):
The root-mean-square molecular speed (
Where,
Answer to Problem 5.136QP
The rms speed of neon gas at
The rms speed of hydrogen gas at
Explanation of Solution
Given,
The given graph represents the distribution of molecular speeds of hydrogen and neon at
Calculation of rms speed:
The rms speed of neon is calculated as follows,
The rms speed of hydrogen gas is calculated as follows,
The rms speed of neon gas at
The rms speed of hydrogen gas at
(c)
Interpretation:
The gas that has greater effusion rate at
Concept Introduction:
Graham’s law of effusion:
Answer to Problem 5.136QP
The gas that has greater effusion rate at
Explanation of Solution
Given,
The given graph represents the distribution of molecular speeds of hydrogen and neon at
Gas possessing greater effusion rate:
The rates of effusion are directly related to the rms speed.
Greater the rms speed, greater is the rate of effusion.
Since the rms speed of hydrogen is greater than neon, hydrogen gas will have greater effusion rate.
In the container, the fast moving molecules collide with the holes of the container more often and hence have a higher effusion probability.
The gas that has greater effusion rate at
(d)
Interpretation:
The temperature at which the rms speed of the hydrogen gas equals the rms speed of neon gas at
Concept Introduction:
Root-mean-square (rms):
The root-mean-square molecular speed (
Where,
Answer to Problem 5.136QP
The temperature at which the rms speed of the hydrogen gas equals the rms speed of neon gas at 200 K is
Explanation of Solution
Given,
The given graph represents the distribution of molecular speeds of hydrogen and neon at
Temperature calculation:
The temperature equaling the rms speed of neon is calculated from root mean square equation as follows,
The temperature at which the rms speed of the hydrogen gas equals the rms speed of neon gas at
Want to see more full solutions like this?
Chapter 5 Solutions
General Chemistry - Standalone book (MindTap Course List)
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
Organic Chemistry
General, Organic, and Biological Chemistry - 4th edition
Organic Chemistry (8th Edition)
- A compound has the molecular formula CH40, and shows a strong IR absorption at 2850-3150 cm. The following signals appear in the 'H NMR spectrum: 1.4 ppm (triplet, 6H), 4.0 ppm (quartet, 4H), 6.8 ppm (broad singlet, 4H). Which of the following structures is consistent with these data? Select the single best answer. OCH CH₂ x OCH2CH3 CH₂OCH3 OH CH₂OCH OH CH, OCH₁ CH₂OCH, CH₂OCH HO OH ° CH₂OCH3arrow_forwardpredict the major product while showing me the intermidiate products from each reagent/reagent grouparrow_forwardWhy is it desirable in the method of standard addition to add a small volume of concentrated standard rather than a large volume of dilute standard? An unknown sample of Cu2+ gave an absorbance of 0.262 in an atomic absorption analysis. Then 1.00 mL of solution containing 100.0 ppm (= µg/mL) Cu2+ was mixed with 95.0 mL of unknown, and the mixture was diluted to 100.0 mL in a volumetric flask. The absorbance of the new solution was 0.500. Calculate the concentration of copper ion in the sample.arrow_forward
- What is the relation between the standard deviation and the precision of a procedure? What is the relation between standard deviation and accuracy? The percentage of an additive in gasoline was measured six times with the following results: 0.13, 0.12, 0.16, 0.17, 0.20, 0.11%. Find the 90% and 99% confidence intervals for the percentage of the additive.arrow_forwardIf you measure a quantity four times and the standard deviation is 1.0% of the average, can you be 90% confident that the true value is within 1.2% of the measured average?arrow_forwardWrite down three most common errors in thermogravimetric analysis. Identify them as systematic or random errors and discuss how you can minimize the errors for better results.arrow_forward
- a) A favorable entropy change occurs when ΔS is positive. Does the order of the system increase or decrease when ΔS is positive? (b) A favorable enthalpy change occurs when ΔH is negative. Does the system absorb heat or give off heat when ΔH is negative? (c) Write the relation between ΔG, ΔH, and ΔS. Use the results of parts (a) and (b) to state whether ΔG must be positive or negative for a spontaneous change. For the reaction, ΔG is 59.0 kJ/mol at 298.15 K. Find the value of K for the reaction.arrow_forwardA sample of hydrated magnesium sulfate (MgSO4⋅xH2O) is analyzed using thermogravimetric analysis (TGA). The sample weighs 2.50 g initially and is heated in a controlled atmosphere. As the temperature increases, the water of hydration is released in two stages: (a) The first mass loss of 0.72 g occurs at 150°C, corresponding to the loss of a certain number of water molecules. (b) The second mass loss of 0.90 g occurs at 250°C, corresponding to the loss of the remaining water molecules. The residue is identified as anhydrous magnesium sulfate (MgSO4) Questions: (i) Determine the value of x (the total number of water molecules in MgSO4⋅xH2O) (ii) Calculate the percentage of water in the original sample. Write down the applications of TGA.arrow_forwardThe solubility product of iron(III) hydroxide (Fe(OH)3) is 6.3×10−38. If 50 mL of a 0.001 M FeCl3 solution is mixed with 50 mL of a 0.005 M NaOH solution, will Fe(OH)3 precipitate? Show all step-by-step calculations. To evaluate the equilibrium constant, we must express concentrations of solutes in mol/L, gases in bars, and omit solids, liquids, and solvents. Explain why.arrow_forward
- Predict the major products of this organic reaction.arrow_forward2. Provide the structure of the major organic product in the following reaction. Pay particular attention to the regio- and stereochemistry of your product. H3CO + H CN Aarrow_forwardPredict the major products of the following organic reaction.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





