
Concept explainers
5-118 Isooctane, which has a chemical formula C8H18 is the component of gasoline from which the term octane rating derives.
(a) Write the balanced chemical equation for the combustion of isooctane.
(b) The density of isooctane is 0.792 g/mL. How many kg of C02 are produced each year by the annual U.S. gasoline consumption of 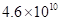 L?
L?
(c) What is the volume in liters of this CO2 at STP?
(d) The chemical formula for isooctane can be represented by (CH3)3CCH2CH(CH3)2. Draw a Lewis structure of isooctane.
(e) Another molecule with the same molecular formula is octane, which can be represented by:
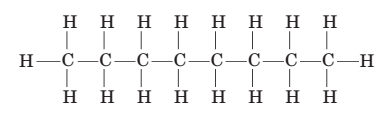
When comparing isooctane and octane, one structure is observed to have a boiling point of 99°C, while another is known to have a boiling point Of 125°C. Which substance, isooctane or octane, is expected to have the higher boiling point?
(f) Determine whether isooctane or octane is expected to have the greater vapor pressure.
(a)
Interpretation:
The balanced chemical equation for the combustion of isooctane should be determined.
Concept Introduction:
In a balanced chemical equation, all the constituents present in the reaction have equal number of atoms on both side of the reaction arrow.
Answer to Problem 5.118P
Explanation of Solution
Isooctane on combustion in the presence of oxygen produces carbon dioxide gas along with water vapor. The balanced chemical equation of the reaction is taking place as depicted below:
(b)
Interpretation:
The mass in kg of CO2 are produced each year by the annual US gasoline consumption should be determined.
Concept Introduction:
Mass of carbon dioxide produces can be obtained from the balanced reaction i.e.
Density of a substance is defined as mass per unit volume. It is mathematically represented as follows:
Here, m is mass and v is volume of the substance.
Answer to Problem 5.118P
Mass of carbon dioxide produces yearly is
Explanation of Solution
Volume of gasoline consumed in 1 year is 4.6 × 1010 L.
Density of the fuel = 0.792 g/mL.
To calculate mass of gasoline, multiply volume of gasoline consumed with its density.
Therefore, the mass of gasoline = 3.64 × 1013 g.
The balanced combustion reaction of gasoline is as follows.
From the balanced reaction,
To calculate the mass of carbon dioxide produces from the
To convert the mass of carbon dioxide from g to kg as follows:
Therefore, mass of carbon dioxide produces yearly is
(c)
Interpretation:
The volume of CO2 in liter at STP should be determined.
Concept Introduction:
Volume CO2 in liter at STP can be determined by calculating moles of CO2 and then using the concept that at STP for each mode of gas occupies 22.4 L.
Answer to Problem 5.118P
The volume of carbon dioxide occupied is
Explanation of Solution
To calculate the number of the moles of carbon dioxide, divide mass of carbon dioxide with its molar mass.
At STP for each mode of gas occupies 22.4 L.
Calculate the volume of carbon dioxide occupied by
Therefore, the volume of carbon dioxide occupied is
(d)
Interpretation:
The Lewis structure of isooctane should be drawn.
Concept Introduction:
Lewis structure are diagrams which represents the bonding between atoms of a molecule and the lone pairs of electrons that might be present in the molecule.
Answer to Problem 5.118P
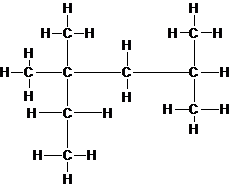
Explanation of Solution
The lewis structure of isooctane is shown in the following diagram. Since, valence electrons in carbon atom are 4 and all the carbon atoms are attached with 4 other atoms (carbon and hydrogen), they have complete octets and all the electrons are engaged in bonding. Thus, there is no lone pair present on any atom.
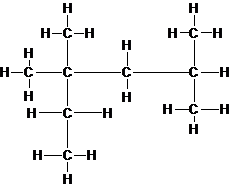
(e)
Interpretation:
If isooctane or octane is expected to have the higher boiling point should be determined.
Concept Introduction:
One of the parameter for specific boiling point of any liquid can be explained by van der waals bonds.
Liquid having more van der waals bonds will show higher boiling point.
Answer to Problem 5.118P
Isooctance has fewer cohensive interactions than octane so Isooctance has lower boiling point.
Explanation of Solution
Van der Waals attractions increase with the surface areas of the interacting electron clouds. That is, the large the interacting surfaces, the greater the magnitude of the induced dipole. Because isooctance has less surface area at which van der waals interactions with other isooctane molecules can occur, it has fewer cohensive interactions than octane, and thus, a lower boiling point.
(f)
Interpretation:
If isooctane or octane is expected to have the greater vapor pressure should be determined.
Concept Introduction:
Vapor pressure depends on boiling point. lower boiling point shows higher vapor pressure.
Answer to Problem 5.118P
Isooctane is expected to have higher vapour pressure at certain temperature.
Explanation of Solution
As the boiling point of isooctane is lower than Octane, isooctane is expected to have higher vapour pressure at certain temperature.
Want to see more full solutions like this?
Chapter 5 Solutions
Introduction to General, Organic and Biochemistry
- Experiment 27 hates & Mechanisms of Reations Method I visual Clock Reaction A. Concentration effects on reaction Rates Iodine Run [I] mol/L [S₂082] | Time mo/L (SCC) 0.04 54.7 Log 1/ Time Temp Log [ ] 13,20] (time) / [I] 199 20.06 23.0 30.04 0.04 0.04 80.0 22.8 45 40.02 0.04 79.0 21.6 50.08 0.03 51.0 22.4 60-080-02 95.0 23.4 7 0.08 0-01 1970 23.4 8 0.08 0.04 16.1 22.6arrow_forward(15 pts) Consider the molecule B2H6. Generate a molecular orbital diagram but this time using a different approach that draws on your knowledge and ability to put concepts together. First use VSEPR or some other method to make sure you know the ground state structure of the molecule. Next, generate an MO diagram for BH2. Sketch the highest occupied and lowest unoccupied MOs of the BH2 fragment. These are called frontier orbitals. Now use these frontier orbitals as your basis set for producing LGO's for B2H6. Since the BH2 frontier orbitals become the LGOS, you will have to think about what is in the middle of the molecule and treat its basis as well. Do you arrive at the same qualitative MO diagram as is discussed in the book? Sketch the new highest occupied and lowest unoccupied MOs for the molecule (B2H6).arrow_forwardQ8: Propose an efficient synthesis of cyclopentene from cyclopentane.arrow_forward
- Q7: Use compound A-D, design two different ways to synthesize E. Which way is preferred? Please explain. CH3I ONa NaOCH 3 A B C D E OCH3arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward(10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forward
- Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardQ3: Rank the following compounds in increasing reactivity of E1 and E2 eliminations, respectively. Br ca. go do A CI CI B C CI Darrow_forward
- Q5: Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2). H₂O דיי "Br KN3 CH3CH2OH NaNH2 NH3 Page 3 of 6 Chem 0310 Organic Chemistry 1 HW Problem Sets CI Br excess NaOCH 3 CH3OH Br KOC(CH3)3 DuckDuckGarrow_forwardQ4: Circle the substrate that gives a single alkene product in a E2 elimination. CI CI Br Brarrow_forwardPlease calculate the chemical shift of each protonsarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





