
Concept explainers
5-16 Answer true or false.
(a) For a sample of gas at constant temperature, its pressure multiplied by its volume is a constant.
(b) For a sample of gas at constant temperature, increasing the pressure increases the volume.
(c) For a sample of gas at constant temperature, 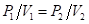
(d) As a gas expands at constant temperature, its volume increases.
(e) The volume of a sample of gas at constant pressure is directly proportional to its temperature—the higher its temperature, the greater its volume.
(f) A hot-air balloon rises because hot air is less dense than cooler air.
(g) For a gas sample in a container of fixed volume, an increase in temperature results in an increase in pressure.
(h) For a gas sample in a container of fixed volume, 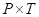 is a constant.
is a constant.
(i) When steam at 100°C in an autoclave is heated to 1200C, the pressure within the autoclave increases.
(j) When a gas sample in a flexible container at constant pressure at 25°C is heated to 50°C, its volume doubles.
(k) Lowering the diaphragm causes the chest cavity to increase in volume and the pressure of air in the lungs to decrease.
(l) Raising the diaphragm decreases the volume of the chest cavity and forces air out of the lungs.
(a)
Interpretation:
Find if the given statement is true or false.
“For a sample of gas at constant temperature, its pressure multiplied by its volume is a constant.”
Concept Introduction:
According to Boyle’s Law, the volume of fixed amount of gas is inversely proportional to the pressure of the gas at constant temperature. Mathematically, it is given as.
Answer to Problem 5.16P
For a sample of gas at constant temperature, its pressure multiplied by its volume is a constant, the given statement is true.
Explanation of Solution
Given information:
For a sample of gas at constant temperature, its pressure multiplied by its volume is a constant.
We know, according to Boyle’s Law, we have.
Thus, for a sample of gas at constant temperature, its pressure multiplied by its volume is a constant.
(b)
Interpretation:
Find if the given statement is true or false.
“For a sample of gas at constant temperature, increasing the pressure increases the volume.”
Concept Introduction:
According to Boyle’s Law, the volume of fixed amount of gas is inversely proportional to the pressure of the gas at constant temperature. Mathematically, it is given as.
Answer to Problem 5.16P
For a sample of gas at constant temperature, increasing the pressure decreases the volume thus, the given statement is false.
Explanation of Solution
Given Information:
For a sample of gas at constant temperature, increasing the pressure increases the volume.
We know, according to Boyle’s Law, we have.
That means there is an inverse relationship between volume and pressure at constant temperature. When pressure is increased, volume is decreased and vice versa.
Thus, for a sample of gas at constant temperature, increasing the pressure decreases the volume.
(c)
Interpretation:
Find if the given statement is true or false.
“For a sample of gas at constant temperature,
Concept Introduction:
According to Boyle’s Law, the volume of fixed amount of gas is inversely proportional to the pressure of the gas at constant temperature. Mathematically, it is given as.
Answer to Problem 5.16P
For a sample of gas at constant temperature,
Explanation of Solution
Given Information:
For a sample of gas at constant temperature,
We know, according to Boyle’s Law, we have.
We two different sets of volume and pressure of the gas is considered, the above equation becomes as follows:
where
Thus, for a sample of gas at constant temperature,
(d)
Interpretation:
Find if the given statement is true or false.
“As a gas expands at constant temperature, its volume increases.”
Concept Introduction:
According to Boyle’s Law, the volume of fixed amount of gas is inversely proportional to the pressure of the gas at constant temperature. Mathematically, it is given as.
Answer to Problem 5.16P
As a gas expands at constant temperature, its volume increases. Thus, the given statement is true.
Explanation of Solution
Given Information:
As a gas expands at constant temperature, its volume increases.
When a gas expands, the distance between the gas particles increases. Thus, pressure decreases.
Also, we know, according to Boyle’s Law.
Decrease in pressure increases the volume.
Thus, as a gas expands at constant temperature, its volume increases.
(e)
Interpretation:
Find if the given statement is true or false.
“The volume of a gas at constant pressure is directly proportional to its temperature − the higher its temperature, the greater its volume.”
Concept Introduction:
According to Charles’s Law, for the gas held at constant pressure, the volume of gas is directly proportional to the temperature of the gas. Mathematically, it is given as.
Answer to Problem 5.16P
The volume of a gas at constant pressure is directly proportional to its temperature − the higher its temperature, the greater its volume. Thus, the given statement is true.
Explanation of Solution
Given Information:
The volume of a gas at constant pressure is directly proportional to its temperature − the higher its temperature, the greater its volume.
We know, according to Charles’s Law, we have.
There is a direct relationship between volume and temperature. As the temperature is increased, the volume is also increased.
Thus, the volume of a gas at constant pressure is directly proportional to its temperature − the higher its temperature, the greater its volume.
(f)
Interpretation:
Find if the given statement is true or false.
“A hot-air balloon rises because hot air is less dense than cooler air”.
Concept Introduction:
Hot air rises because when the air is heated, it undergoes expansion. This results in the air to become less dense than the air surrounding it.
Answer to Problem 5.16P
A hot-air balloon rises because hot air is less dense than cooler air, thus, the given statement is true.
Explanation of Solution
Given information:
A hot-air balloon rises because hot air is less dense than cooler air.
Hot air rises because when the air is heated, it undergoes expansion. This results in the air to become less dense than the air surrounding it.
Thus, a hot-air balloon rises because hot air is less dense than cooler air.
(g)
Interpretation:
Find if the given statement is true or false.
“For a gas sample in a container of fixed volume, an increase in temperature results in the increase in pressure.”
Concept Introduction:
According to Gay-Lussac’s Law, for the gas held at constant volume, the pressure of a given amount of gas is directly proportional to the temperature of the gas. Mathematically, it is given as.
Answer to Problem 5.16P
For a gas sample in a container of fixed volume, an increase in temperature results in the increase in pressure. Thus, the given statement is true.
Explanation of Solution
Given information:
For a gas sample in a container of fixed volume, an increase in temperature results in the increase in pressure.
We know, according to Gay Lussac’s Law, we have.
There is a direct relationship between pressure and temperature. As the temperature is increased, the pressure is also increased.
Thus, for a gas sample in a container of fixed volume, an increase in temperature results in the increase in pressure.
(h)
Interpretation:
Find if the given statement is true or false.
“For a gas sample in a container of fixed volume,
Concept Introduction:
According to Gay-Lussac’s Law, for the gas held at constant volume, the pressure of a given amount of gas is directly proportional to the temperature of the gas. Mathematically, it is given as.
Answer to Problem 5.16P
For a gas sample in a container of fixed volume,
Explanation of Solution
Given information:
For a gas sample in a container of fixed volume, an increase in temperature results in the increase in pressure.
We know, according to Gay Lussac’s Law, we have.
Thus, for a gas sample in a container of fixed volume,
(i)
Interpretation:
Find if the given statement is true or false.
“When steam at
Concept Introduction:
According to Gay-Lussac’s Law, for the gas held at constant volume, the pressure of a given amount of gas is directly proportional to the temperature of the gas. Mathematically, it is given as.
Answer to Problem 5.16P
When steam at
Explanation of Solution
Given Information:
When steam at
We know, according to Gay Lussac’s Law, we have.
There is a direct relationship between pressure and temperature. As the temperature is increased, the pressure is also increased.
Thus, when steam at
(j)
Interpretation:
Find if the given statement is true or false.
“When a gas sample in a flexible container at constant pressure at
Concept Introduction:
According to Charles’s Law, for the gas held at constant pressure, the volume of gas is directly proportional to the temperature of the gas. Mathematically, it is given as.
Answer to Problem 5.16P
When a gas sample in a flexible container at constant pressure at
Explanation of Solution
Given Information:
When a gas sample in a flexible container at constant pressure at
We know, according to Charles’s Law, we have.
There is a direct relationship between volume and temperature. As the temperature is increased, the volume is also increased.
Now, the temperature of the container is doubled, hence the volume of the flexible container is also doubled.
Thus, when a gas sample in a flexible container at constant pressure at
(k)
Interpretation:
Find if the given statement is true or false.
“Lowering the diaphragm causes the chest cavity to increase in volume and the pressure of air in the lungs to decrease”.
Concept Introduction:
According to Boyle’s Law, the volume of fixed amount of gas is inversely proportional to the pressure of the gas at constant temperature. Mathematically, it is given as.
Answer to Problem 5.16P
Lowering the diaphragm causes the chest cavity to increase in volume and the pressure of air in the lungs to decrease thus, the given statement is true.
Explanation of Solution
Given information:
Lowering the diaphragm causes the chest cavity to increase in volume and the pressure of air in the lungs to decrease.
We know, according to Boyle’s Law, we have.
Now, when diaphragm is lowered, the chest cavity is increased. This results in the increase in volume and hence pressure decreases inside the lungs.
(l)
Interpretation:
Find if the given statement is true or false.
“Raising the diaphragm decreases the volume of the chest cavity and forces air out of the lungs.”
Concept Introduction:
According to Boyle’s Law, the volume of fixed amount of gas is inversely proportional to the pressure of the gas at constant temperature. Mathematically, it is given as.
Answer to Problem 5.16P
Raising the diaphragm decreases the volume of the chest cavity and forces air out of the lungs. Thus, the given statement is true.
Explanation of Solution
Given information:
Raising the diaphragm decreases the volume of the chest cavity and forces air out of the lungs.
We know, according to Boyle’s Law, we have.
Now, when diaphragm is raised, the volume chest cavity is decreased. This results in the decrease in volume and hence pressure increased inside the lungs and air is moved out of the lungs.
Want to see more full solutions like this?
Chapter 5 Solutions
Introduction to General, Organic and Biochemistry
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forwardLook at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forward
- Given 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- Concentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forwardExplain why the following names of the structures are incorrect. CH2CH3 CH3-C=CH-CH2-CH3 a. 2-ethyl-2-pentene CH3 | CH3-CH-CH2-CH=CH2 b. 2-methyl-4-pentenearrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





