
Microbiology with Diseases by Body System & Modified MasteringMicrobiology with Pearson eText -- ValuePack Access Card -- for Microbiology with Diseases by Body System Package
1st Edition
ISBN: 9780133857122
Author: Robert W. Bauman Ph.D.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 3VI
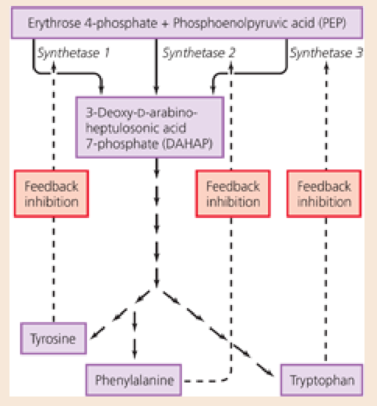
Examine the biosynthetic pathway for the production of the amino acids tryptophan, tyrosine, and phenylalanine in the figure. Where do the initial reactants (erythrose4-phosphate and PEP) originate?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Hemoglobin is the oxygen carrier in the blood. What is the effect of each of the following treatments on the oxygen affinity of hemoglobin in vitro and how do these changes impact the T state vs. R state of the enzyme?
A) Decrease in pH from 7.4 to 7.2.
B) Increase in 2,3-BPG concentration.
(b)( ) In mature erythrocytes (red blood
cells) the end product of glycolysis is lactate
because of the absence of mitochondria. On
the right is a table comparing the rate of lac-
tate production in hemolysates (lysed cells)
of human RBCs as a function of pH with dif-
ferent substrates introduced into the glyco-
lytic pathway. The hemolysate was fortified
with 30 μmoles substrate, 7.5 μmoles MgCl2,
10 μmoles disodium phosphate, 1.5 μmoles
NAD* and 5 μmoles ATP in a volume of 5
TABLE 3-LACTATE PRODUCTION IN FORTIFIED HEMOLYSATES OF HUMAN
ERYTHROCYTES*
Substrate
Glucose
Glucose
Glucose-6-phosphate
Glucose-6-phosphate
Fructose-1,6-diphosphate
Fructose-1,6-diphosphate
Lactate
production†
No. of
experiments
pH
6
7.1
2.03 ± 0.91
6
7.8
4.76 ± 1.09
5
7.1
10-731-88
5
7.8
12.34 ±2.92
5
7.0
7.15±0.73
5
7.7
7.15±0.80
mL. The rate of lactate production is given as μmoles of lactate/g Hb/hr at 37° C, buffered to either pH
7.1 or 7.8, as indicated. According to the results in the table which…
Name three enzymes that are likely the source of bicarbonate ion that is involved with the formation of carbamoyl phosphate?
In the Ubiquitin pathway, why are there many more E3 proteins than E1 and E2 combined?
Chapter 5 Solutions
Microbiology with Diseases by Body System & Modified MasteringMicrobiology with Pearson eText -- ValuePack Access Card -- for Microbiology with Diseases by Body System Package
Ch. 5 - How can oxidation take place in an anaerobic...Ch. 5 - Why do electrons carried by NADH allow for...Ch. 5 - Why does catabolism of amino acids for energy...Ch. 5 - An uninformed student describes the Calvin-Benson...Ch. 5 - Prob. 5TMWCh. 5 - Why is feedback inhibition necessary for...Ch. 5 - Breaks a large molecule into smaller ones a....Ch. 5 - Includes dehydration synthesis reactions a....Ch. 5 - Prob. 3MCCh. 5 - Prob. 4MC
Ch. 5 - Involves the production of cell membrane...Ch. 5 - Includes hydrolytic reactions a. anabolism only b....Ch. 5 - Includes metabolism a. anabolism only b. both...Ch. 5 - Prob. 8MCCh. 5 - A reduced molecule _________. a. has gained...Ch. 5 - Prob. 10MCCh. 5 - Coenzymes are ________. a. types of apoenzymes b....Ch. 5 - Which of the following statements best describes...Ch. 5 - Which of the following does not affect the...Ch. 5 - Most oxidation reactions in bacteria involve the...Ch. 5 - Under ideal conditions, the fermentation of one...Ch. 5 - Under ideal conditions, the complete aerobic...Ch. 5 - Which of the following statements about the...Ch. 5 - Reactions involved in the light-independent...Ch. 5 - The glycolysis pathway is basically __________. a....Ch. 5 - A major difference between anaerobic respiration...Ch. 5 - 1. _______ Occurs when energy from a compound...Ch. 5 - Fill in the Blanks 1. The final electron acceptor...Ch. 5 - Fill in the Blanks 2. Two ATP molecules are used...Ch. 5 - Fill in the Blanks 3. The initial catabolism of...Ch. 5 - Fill in the Blanks 4. ________ is a cyclic series...Ch. 5 - Fill in the Blanks 5. The final electron acceptor...Ch. 5 - Fill in the Blanks 6. Three common inorganic...Ch. 5 - Fill in the Blanks 7. Anaerobic respiration...Ch. 5 - Fill in the Blanks 8. Complete the following...Ch. 5 - Prob. 9FIBCh. 5 - Fill in the Blanks 10 The main coenzymes that...Ch. 5 - VISUALIZE IT! 1 Label the mitochondrion to...Ch. 5 - Label the diagram below to indicate acetyl-CoA,...Ch. 5 - Examine the biosynthetic pathway for the...Ch. 5 - Prob. 1SACh. 5 - Why we enzymes necessary for anabolic reactions to...Ch. 5 - How do organisms control the rate of metabolic...Ch. 5 - How does a nor-competitive inhibitor at a single...Ch. 5 - Explain the mechanism of negative feedback with...Ch. 5 - Facultative anaerobes can live under either...Ch. 5 - How does oxidation of a molecule occur without...Ch. 5 - List at least four groups of microorganisms that...Ch. 5 - Why do we breathe oxygen and give of carbon...Ch. 5 - Why do cyanobacteria and algae take in carbon...Ch. 5 - What happens to the carbon atoms in sugar...Ch. 5 - How do yeast cells make alcohol and cause bread to...Ch. 5 - Where specifically does the most significant...Ch. 5 - Why are vitamins essential metabolic factors for...Ch. 5 - A laboratory scientist notices that a cer1ain...Ch. 5 - Arsenic is a poison that exists in two states in...Ch. 5 - Explain why an excess of all three of the amino...Ch. 5 - Why might an organism that uses glycolysis and the...Ch. 5 - Describe how bacterial fermentation causes milk to...Ch. 5 - Giardia intestinalis and Entamoeba histolytica are...Ch. 5 - Two cultures of a facultative anaerobe are grown...Ch. 5 - What is the maximum number of molecules of ATP...Ch. 5 - In terms of its effects on human metabolism, why...Ch. 5 - Cyanide is a potent poison because it irreversibly...Ch. 5 - How are photophosphorylation and oxidative...Ch. 5 - Members of the pathogenic bacterial genus...Ch. 5 - Compare and contrast aerobic respiration,...Ch. 5 - Scientists estimate that up to one-third of Earths...Ch. 5 - A young student was troubled by the idea that a...Ch. 5 - If a bacterium uses beta-oxidation to catabolize a...Ch. 5 - Some desert rodents rarely have water to drink....Ch. 5 - Prob. 17CTCh. 5 - We have examined the total ATP, NADH, and FADH2...Ch. 5 - Explain why hyperthermophiles do not cause disease...Ch. 5 - In addition to extremes in temperature and pH,...Ch. 5 - Figure 5.18b illustrates events in aerobic...Ch. 5 - Suppose you could insert a tiny pH probe into the...Ch. 5 - Even though Pseudomonas aeruginosa and...Ch. 5 - Photosynthetic organisms are rarely pathogenic....Ch. 5 - Prob. 25CTCh. 5 - A scientist moves a green plant grown in sunlight...Ch. 5 - What class of enzyme is involved in amination...Ch. 5 - Using the following terms, fill in the following...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Phosphofructokinase 1 (PFK1) activity is regulated by the concentration of ATP through allostery. Increasing the cellular concentration of ATP decreases the affinity of the enzyme for the substrate fructose-6-phosphate. Given that ATP is also one of the substrates for PFK1, how does this regulation mechanism work so effectively? a) ATP can bind to two places on PFK, when ATP is bound in the other site, PFK changes conformation leading to lower affinity for fructose-6-phosphate b) At high concentrations, ATP binds and reverses the PFK reaction c) ATP decreases the activity of aldolase, leading to product inhibition of PFK d) ATP at high concentration binds in place of fructose-6-phosphatearrow_forwardDescribe the two models that explain the binding of allosteric enzymes. Use either model to explain the binding ofoxygen to hemoglobin.arrow_forwardReferring to figure 5 and 8 which substrate molecule serves as the phosphate donor during substrate-level phosphorylation in step 10 of glycosis and the succinyl CoA-succinate step of the Krebs Cycle?arrow_forward
- Identify the substrate and products of the TCA cycle. Describe its organization in general terms. What are its major functions?arrow_forwardWhat compound(s) were the most effective inhibitors and what compound(s) were the most effective activators?arrow_forwardRed blood cells synthesize and degrade 2,3-biphosphogylerate (2,3-BPG) as a detour from the glycolytic pathway, as shown in the figure.2,3-BPG decreases the oxygen affinity of hemoglobin by binding in the central cavity of the deoxygenated form of hemoglobin. This encourages delivery of oxygen to tissues. A defect in one of the glycolytic enzymes may affect levels of 2,3-BPG. The plot above right shows oxygen-binding curves for normal erythrocytes and for hexokinase and pyruvate kinase-deficient erythrocytes. Identify which curve corresponds to which enzyme deficiency.arrow_forward
- a) Based on the mechanism shown in Figure 2A, what type of enzyme is transpeptidase? : Lyase Isomerase Ligase Hydrolase Oxidoreductase Transferase b) Transpeptidases have two substrates. From Figure 2A, what type of mechanism do they most likely adopt in processing the two substrates? sequential or ping-pong c) β-lactams inactivate transpeptidases by forming a covalent bond with the serine residue in the active site. Based on this description and Figure 2B caption, what type of inhibitor are β-lactams? _________________________________________ d) Based on the mechanism for lactamase shown in Figure 3, what type of enzyme is lactamase? Lyase Isomerase Ligase Hydrolase Oxidoreductase Transferase e) Based on your answer in d, what other reactant, in addition to the antibiotic substrate, needs to be in the active site of lactamase for the hydrolysis reaction to proceed? ____________________arrow_forwardIn the liver, what is the major regulation that dictates fate of pyruvate vs lactate? (This can tie into reading assignment!) a) Activation of MCT1 to transport lactate out of cell b) Activation of pyruvate carboxylase and conc dictating use of lactate dehydrogenase, inhibition of PDHC c) Activation of lactate dehydrogen, inactivation of pyruvate carboxylasease and PDHC d) Activation of lactate dehydrogenase and MCT1 (transporter)arrow_forwardPeroxidase is an enzyme found in many organisms, from plant to humans. The function of peroxidase is to break down hydrogen peroxide, which is a toxin produced as a byproduct when oxygen is produced during respiration. Myeloperoxidase is a protein found in neutrophils and catalyzes lipid peroxidation involved in immune defense. The overproduction of myeloperoxidase has been found to function as a mediator for tissue damage in inflammatory diseases. (8 points) a. Identify two examples of environmental factors that may impact myeloperoxidase activity. Describe how each of the environmental factors would affect the reaction rate of the enzyme. b. Predict the how researchers can use myeloperoxidase activity as oxidative stress biomarkers in patients with rheumatoid arthritis. Provide reasoning to justify your prediction.arrow_forward
- Identify the following statements as true or false. If a statement is false, explain what is wrong with that statement a) Transition state analogs bind very tightly with the enzyme active site as they form covalent bonds with the active site b) Receptor desensitization happens upon prolonged exposure of receptors to both agonists and antagonists c) Dactinomycin and Doxorubicin are examples of drugs that bind with the major groove of DNA d) Mitomycin C forms interstrand crosslinking while cisplatin forms intrastrand crosslinking of DNAarrow_forwardWhat would be the metabolic consequences and symptoms of having a mutated form of phosphofructokinase-1 in muscle that is no longer allosterically regulated by [H+]? Speculate on how a patient with this mutation could deal with this.arrow_forwardBriefly describe the role of nucleophilic catalysis in the mechanism of the chymotrypsin reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education
Anaerobic Respiration; Author: Bozeman Science;https://www.youtube.com/watch?v=cDC29iBxb3w;License: Standard YouTube License, CC-BY