
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 23E
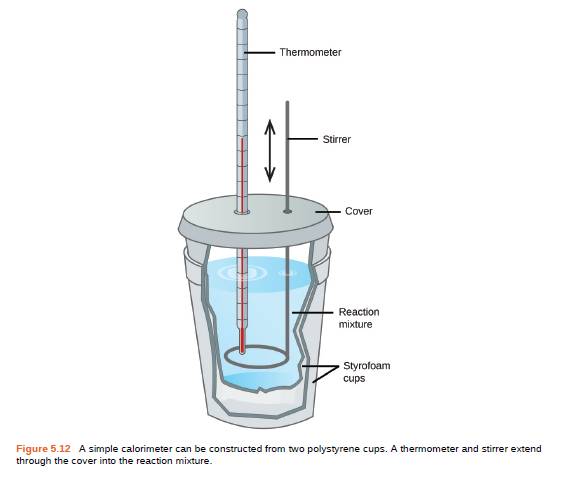
If a reaction produces 1.506 kJ of heat, which is trapped in 30.0 g of water initially at 26.5 °C in a calorimeter like that in Figure 5.12, what is the resulting temperature of the water?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Primary, Secondary, and
Tertiary Alcohols
O-H
O-H
O-H
R₁-C-H
R₁-C-H
R₁-C-R₁
H
R₂
R₂
Primary
Alcohol
Secondary
Alcohol
ChemistryLearner.com
R stands for Carbon group like ethyl methyl propyl
Tertiary
Alcohol
If 1 carbon group with two H attached to alcoholic carbon, then primary
If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary
IF 3 carbon group and no H attach to alcoholic carbon then tertiary.
The bottom line
Starting
"Weak" oxidant
material
PCC, DMP, Swern, etc
Primary alcohol
Aldehyde
OH
Secondary alcohol
Ketone
OH
"Strong" oxidant
KMnO4, H₂CrO4
(or equivalent)
OH
Carboxylic acid
요
Ketone
No reaction
No reaction
Tertiary alcohol
1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the
structures of
ethanol and any oxidation products of ethanol. If there is more than one
oxidation product, give the structure of each of the products.
2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the
structures of
2-propanol and any…
Formulate the reaction: Naphthalene with CrO3 in acetic acid at 25ºC
Complete the reaction
hand written please
Chapter 5 Solutions
Chemistry by OpenStax (2015-05-04)
Ch. 5 - A burning match and a bonfire may have the same...Ch. 5 - Prepare a table identifying several energy...Ch. 5 - Explain the difference between heat capacity and...Ch. 5 - Calculate the heat capacity, in joules and in...Ch. 5 - Calculate the heat capacity, in joules and in...Ch. 5 - How much heat, in joules and in calories, must be...Ch. 5 - How much heat, in joules and in calories, is...Ch. 5 - How much would the temperature of 275 g of water...Ch. 5 - If 14.5 kJ of heat were added to 485 g of liquid...Ch. 5 - A piece of unknown substance weighs 44.7 g and...
Ch. 5 - A piece of unknown solid substance weighs 437.2 g,...Ch. 5 - An aluminum kettle weighs 1.05 kg. (a) What is the...Ch. 5 - Most people find waterbeds uncomfortable unless...Ch. 5 - A 500-mL bottle of water at room temperature and a...Ch. 5 - Would the amount of heat measured for the reaction...Ch. 5 - Would the amount of heat absorbed by the...Ch. 5 - Would the amount of heat absorbed by the...Ch. 5 - How many milliliters of water at 23 C with a...Ch. 5 - How much will the temperature of a cup (180 g) of...Ch. 5 - A 45-g aluminum spoon (specific heat 0.88 J/g C)...Ch. 5 - The temperature of the cooling water as it leaves...Ch. 5 - A 70.0-g piece of metal at 80.0 °C is placed in...Ch. 5 - If a reaction produces 1.506 kJ of heat, which is...Ch. 5 - A 0.500-g sample of KCl is added to 50.0 g of...Ch. 5 - Dissolving 3.0 g of CaCl2(s) in 150.0 g of water...Ch. 5 - When 50.0 g of 0.200 M NaCl(aq) at 24.1 C is added...Ch. 5 - The addition of 3.15 g of Ba(OH)28H2O to a...Ch. 5 - The reaction of 50 mL of acid and 50 mL of base...Ch. 5 - If the 3.21 g of NH4NO3 in Example 5.6 were...Ch. 5 - When 1.0 g of fructose, C6H12O6(s), a sugar...Ch. 5 - When a 0.740-g sample of trinitrotoluene (TNT),...Ch. 5 - One method of generating electricity is by burning...Ch. 5 - The amount of fat recommended for someone with a...Ch. 5 - A teaspoon of the carbohydrate sucrose (common...Ch. 5 - What is the maximum mass of carbohydrate in a 6-oz...Ch. 5 - A pint of premium ice cream can contain 1100...Ch. 5 - A serving of a breakfast cereal contains 3 g of...Ch. 5 - Which is the least expensive source of energy in...Ch. 5 - Explain how the heat measured in Example 5.5...Ch. 5 - Using the data in the check your learning section...Ch. 5 - Calculate the enthalpy of solution ( H for the...Ch. 5 - Calculate H for the reaction described by the...Ch. 5 - Calculate the enthalpy of solution ( H for the...Ch. 5 - Although the gas used in an oxyacetylene torch...Ch. 5 - How much heat is produced by burning 4.00 moles of...Ch. 5 - How much heat is produced by combustion of 125 g...Ch. 5 - How many moles of isooctane must be burned to...Ch. 5 - What mass of carbon monoxide must be burned to...Ch. 5 - When 2.50 g of methane burns in oxygen, 125 kJ of...Ch. 5 - How much heat is produced when loo mL of 0.250 M...Ch. 5 - A sample of 0.562 g of carbon is burned in oxygen...Ch. 5 - Before the introduction of chlorofluorocarbons,...Ch. 5 - Homes may be heated by pumping hot water through...Ch. 5 - Which of the enthalpies of combustion in Table 5.2...Ch. 5 - Does the standard enthalpy of formation of H2O(g)...Ch. 5 - Joseph Priestly prepared oxygen in 1774 by heating...Ch. 5 - How many kilojoules of heat will be released when...Ch. 5 - How many kilojoules of heat will be released when...Ch. 5 - The following sequence of reactions occurs in the...Ch. 5 - Both graphite and diamond burn....Ch. 5 - From the molar heats of formation in Appendix G,...Ch. 5 - Which produces more heat?...Ch. 5 - Calculate H298 for the process...Ch. 5 - Calculate H298 for the process...Ch. 5 - Calculate H for the process Hg2Cl2(s)2Hg(l)+Cl2(g)...Ch. 5 - Calculate H298 for the process...Ch. 5 - Calculate the standard molar enthalpy of formation...Ch. 5 - Using the data in Appendix G, calculate the...Ch. 5 - Using the data in Appendix G, calculate the...Ch. 5 - The following reactions can be used to prepare...Ch. 5 - The decomposition of hydrogen peroxide, H2O2, has...Ch. 5 - Calculate the enthalpy of combustion of propane,...Ch. 5 - Calculate the enthalpy of combustion of butane,...Ch. 5 - Both propane and butane are used as gaseous fuels....Ch. 5 - The white pigment TiO2 is prepared by the reaction...Ch. 5 - Water gas, a mixture of H2 and CO, is an important...Ch. 5 - In the early days of automobiles, illumination at...Ch. 5 - From the data in Table 5.2, determine which of the...Ch. 5 - The enthalpy of combustion of hard coal averages...Ch. 5 - Ethanol, C2H5OH, is used as a fuel for motor...Ch. 5 - Among the substances that react with oxygen and...Ch. 5 - How much heat is produced when 1.25 g of chromium...Ch. 5 - Ethylene, C2H2, a byproduct from the fractional...Ch. 5 - The oxidation of the sugar glucose, C6H12O6, is...Ch. 5 - Propane, C3H8, is a hydrocarbon that is commonly...Ch. 5 - During a recent winter month in Sheboygan,...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Explain why 92% of 2,4-pemtanedione exists as the enol tautomer in hexane but only 15% of this compound exists ...
Organic Chemistry (8th Edition)
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
1. Why is the quantum-mechanical model of the atom important for understanding chemistry?
Chemistry: Structure and Properties (2nd Edition)
1. ___ Mitosis 2. ___ Meiosis 3. __ Homologous chromosomes 4. __ Crossing over 5. __ Cytokinesis A. Cytoplasmic...
Microbiology with Diseases by Body System (5th Edition)
Police Captain Jeffers has suffered a myocardial infarction. a. Explain to his (nonmedically oriented) family w...
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 3+ 3Cu²+ (aq) +2Al(s) → 3 Cu(s)+2A1³* (aq) 2+ Suppose the cell is prepared with 5.29 M Cu in one half-cell and 2.49 M A1³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. x10 μ ☑ 00. 18 Ar Иarrow_forwardPlease help me solve this homework problemarrow_forwardPlease help me answer this homework questionarrow_forward
- Calculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3+ H2(g)+2OH¯ (aq) + 2Fe³+ (aq) → 2H₂O (1)+2Fe²+ (aq) 0 kJ x10 Х ? olo 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 184.2 mL of a 0.7800M solution of dimethylamine ((CH3) NH with a 0.3000M solution of HClO4. The pK₁ of dimethylamine is 3.27. Calculate the pH of the base solution after the chemist has added 424.1 mL of the HClO solution to it. 2 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO 4 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ ? 000 18 Ar 1 Barrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: MnO2 (s)+4H* (aq)+2Cr²+ (aq) → Mn²+ (aq)+2H₂O (1)+2Cr³+ (aq) + 2+ 2+ 3+ Suppose the cell is prepared with 7.44 M H* and 0.485 M Cr²+ in one half-cell and 7.92 M Mn² and 3.73 M Cr³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. ☐ x10 μ Х 5 ? 000 日。arrow_forward
- Calculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. NO (g) +H₂O (1) + Cu²+ (aq) → HNO₂ (aq) +H* (aq)+Cu* (aq) kJ - ☐ x10 x10 olo 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid b An analytical chemist is titrating 116.9 mL of a 0.7700M solution of aniline (C6H5NH2) with a 0.5300M solution of HNO3. The pK of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 184.2 mL of the HNO 3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ 5arrow_forwardQUESTION: Find the standard deviation for the 4 different groups 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5.033 4.044 334.6 268.7 4.706 3.621 305.6 234.4 4.816 3.728 340.0 262.7 4.828 4.496 304.3 283.2 4.993 3.865 244.7 143.6 STDEV = STDEV = STDEV = STDEV =arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY