
Write structural formulas for all the constitutionally isomeric alcohols of molecular
formula
Interpretation:
The structural formulas for the constitutionally isomeric alcohols of molecular formula
Concept Introduction:
Isomers that have the same molecular formula but differ in the way in which different atoms are connected are known as constitutional isomers.
The number of atoms of each element present in the compound, is known as the molecular formula.
When the functional group
Answer to Problem 22P
Solution:
There are 8 constitutionally isomeric alcohols of molecular formula
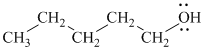
Substitutive name:
Functional class name: pentyl alcohol
It is a primary alcohol.
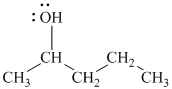
Substitutive name:
Functional class name:
It is a secondary alcohol.
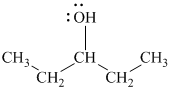
Substitutive name:
Functional class name:
It is a secondary alcohol.
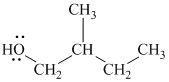
Substitutive name:
Functional class name:
It is a primary alcohol.
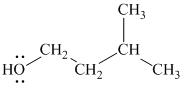
Substitutive name:
Functional class name:
It is a primary alcohol.
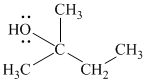
Substitutive name:
Functional class name:
It is a tertiary alcohol.
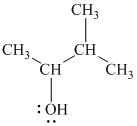
Substitutive name:
Functional class name:
It is a secondary alcohol.
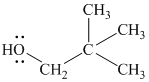
Substitutive name:
Functional class name:
It is a primary alcohol.
Explanation of Solution
In all, there are
They have the same molecular formula but different connectivity of atoms.
The structural formulas of these alcohols can be written as follows.
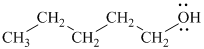
The substitutive name of this alcohol is
The functional class name of this alcohol is pentyl alcohol.
The hydroxyl group is attached to a primary carbon atom. Hence, it is a primary alcohol.
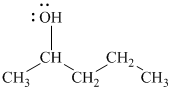
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a secondary carbon atom. Hence, it is a secondary alcohol.
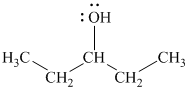
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a secondary carbon atom. Hence, it is a secondary alcohol.
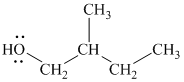
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a primary carbon atom. Hence, it is a primary alcohol.
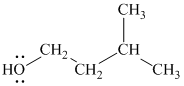
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a primary carbon atom. Hence, it is a primary alcohol.
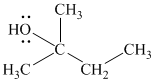
The substitutive name of this alcohol is 2-methyl-2-butanol.
The functional class name of this alcohol is 1,1-dimethylpropanol.
The hydroxyl group is attached to a tertiary carbon atom. Hence, it is a tertiary alcohol.
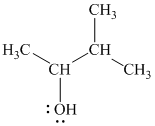
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a secondary carbon atom. Hence, it is a secondary alcohol.
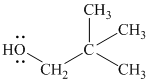
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a primary carbon atom. Hence, it is a primary alcohol.
The structural formulas for the constitutionally isomeric alcohols of molecular formula
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- Determine whether the compound is a primary, secondary, or tertiary alcohol.arrow_forwardName the functional groups for each. Specify primary, secondary, and tertiary alcohols and halides.arrow_forwardExplain using only structural diagrams only the differences between primary,secondary and tertiary alcohols.arrow_forward
- Compounds Y and Z both have the formula C₂H18. Both Y and Z react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methyloctane. The heat of hydrogenation of Y is less than that of Z. Y and Z each undergo hydroboration/oxidation to give a primary alcohol (OH attached to a primary carbon). What is the structure of Y? • In cases where there is more than one answer, just draw one. 1998) 0▾ + n [F ChemDoodle aarrow_forwardHow would you distinguish between primary, secondary and tertiary alcohol in the laboratory?arrow_forwardWrite structural formulas for all ketones with the molecular formula C6H12O and give each its IUPAC name. Which of these ketones are chiral?arrow_forward
- 1 In order to form a tertiary alcohol, excess CH3MgBr could react with which one(s) of these compounds? مل H₂C A CH₂ B CH3 CH3 CH3 H₂C D -OHarrow_forwarda primary alcohol has one -OH group, a secondary hasarrow_forwardGive the structures and IUPAC names for all tertiary alcohols of molecular formula C6H10O.arrow_forward
- Draw Lewis structures and condensed structural formulas for the four alcohols with the molecular formula C4H10O. Classify each alcohol as primary, secondary, or tertiary. (Hint: First consider the connectivity of the four carbon atoms; they can be bonded either four in a chain or three in a chain with the fourth carbon as a branch on the middle carbon. Then consider the points at which the iOH group can be bonded to each carbon chain.)arrow_forwardStarting from an alcohol with no more than 3 carbon atoms, write down all reactions and chemicals step by step to obtain the propylethylet as a single product. (Reactions written other than the reactions you saw in the course will not be accepted.)arrow_forwardThe C-O bond is much shorter in phenol than in ethanol. Give reason.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





