
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
10th Edition
ISBN: 9781260028355
Author: Carey
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 5, Problem 43P
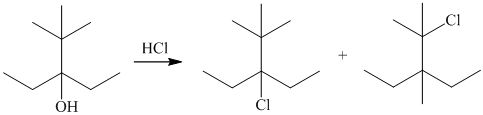
The reaction of
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Construct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)
please help with hw
help me solve this hw
Chapter 5 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
Ch. 5.1 - Prob. 1PCh. 5.1 - Prob. 2PCh. 5.1 - Many compounds contain more than one functional...Ch. 5.2 - Prob. 4PCh. 5.3 - Prob. 5PCh. 5.4 - Classify the isomeric C4H10O alcohols as being...Ch. 5.5 - Bromine is less electronegative than chlorine, yet...Ch. 5.6 - Prob. 8PCh. 5.7 - Prob. 9PCh. 5.8 - Prob. 10P
Ch. 5.8 - Prob. 11PCh. 5.9 - Carbocations are key intermediates in petroleum...Ch. 5.9 - Prob. 13PCh. 5.9 - Prob. 14PCh. 5.11 - Prob. 15PCh. 5.13 - Prob. 16PCh. 5.14 - For the reaction of a primary alcohol RCH2OH with...Ch. 5.15 - Prob. 18PCh. 5 - Write structural formulas for each of the...Ch. 5 - Prob. 20PCh. 5 - Prob. 21PCh. 5 - Write structural formulas for all the...Ch. 5 - Prob. 23PCh. 5 - Prob. 24PCh. 5 - Epichlorohydrin is the common name of an...Ch. 5 - Prob. 26PCh. 5 - Prob. 27PCh. 5 - Prob. 28PCh. 5 - Some of the most important organic compounds in...Ch. 5 - Prob. 30PCh. 5 - Prob. 31PCh. 5 - Prob. 32PCh. 5 - Prob. 33PCh. 5 - Prob. 34PCh. 5 - Prob. 35PCh. 5 - Prob. 36PCh. 5 - Prob. 37PCh. 5 - Prob. 38PCh. 5 - Prob. 39PCh. 5 - Prob. 40PCh. 5 - The reaction of 2,2-dimethyl-1-propanol...Ch. 5 - (a) Assuming that the rate-determining elementary...Ch. 5 - The reaction of 3-tert-butyl-3-pentanol with...Ch. 5 - Prob. 44PCh. 5 - Prob. 45PCh. 5 - Prob. 46DSPCh. 5 - Prob. 47DSPCh. 5 - Prob. 48DSPCh. 5 - Prob. 49DSPCh. 5 - Prob. 50DSPCh. 5 - Prob. 51DSPCh. 5 - Prob. 52DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Briefly explain chemical potential.arrow_forwardReason whether it is possible to determine changes in the Galvani potential difference at the metal-solution interface.arrow_forwardObtain the standard potential at 25°C of the Cu* I Cu | Pt electrode from the standard potentials E° Cu²+/Cu = 0.341 V and E Cu²+ /Cu+ = 0.153 V.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY