
Custom eBook for Organic Chemistry
2nd Edition
ISBN: 9798214171104
Author: Straumanis
Publisher: Cengage Custom
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 19CTQ
The
a. Is this an important resonance structure of acetone? Explain.
b. Does this structure convey any useful information about acetone? If so, what?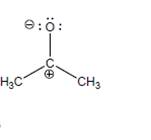
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Hi can you please help me solve these problems? thank you
Hi can you please help me solve this problem? thank you
Hi can you please help me solve this problem? thank you
Chapter 5 Solutions
Custom eBook for Organic Chemistry
Ch. 5 - Which elements on the periodic table (other than...Ch. 5 - You will not find “hydroxide” in the stockroom,...Ch. 5 - Prob. 3CTQCh. 5 - Prob. 4CTQCh. 5 - Prob. 5CTQCh. 5 - Prob. 6CTQCh. 5 - On which do you expect to have a more intense and...Ch. 5 - Prob. 8CTQCh. 5 - Prob. 9CTQCh. 5 - Prob. 10CTQ
Ch. 5 - Prob. 11CTQCh. 5 - Prob. 12CTQCh. 5 - Prob. 13CTQCh. 5 - Prob. 14CTQCh. 5 - Prob. 15CTQCh. 5 - Prob. 16CTQCh. 5 - For each proposed set of resonance structures: a....Ch. 5 - Consider the polarization of the C=O bond in the...Ch. 5 - The C=O double bond is called a “carbonyl bond.”...Ch. 5 - Prob. 20CTQCh. 5 - Prob. 21CTQCh. 5 - Prob. 22CTQCh. 5 - Prob. 23CTQCh. 5 - Prob. 24CTQCh. 5 - Prob. 25CTQCh. 5 - Prob. 26CTQCh. 5 - Prob. 27CTQCh. 5 - Prob. 28CTQCh. 5 - Prob. 29CTQCh. 5 - Prob. 30CTQCh. 5 - Prob. 31CTQCh. 5 - Confirm that there is no legitimate Lewis...Ch. 5 - Draw all resonance structures of the molecule...Ch. 5 - Prob. 34CTQCh. 5 - Prob. 35CTQCh. 5 - Prob. 36CTQCh. 5 - Occasionally, we will see an ionic compound that...Ch. 5 - Prob. 2ECh. 5 - Prob. 3ECh. 5 - Prob. 4ECh. 5 - Is it possible to draw a resonance structure of...Ch. 5 - Prob. 6ECh. 5 - Prob. 7ECh. 5 - Prob. 8ECh. 5 - Phenol (shown below) has a pKa10 . a. Based on pKa...Ch. 5 - Use curved arrows to show the most likely...Ch. 5 - Prob. 12ECh. 5 - Complete each Lewis structure, draw all important...Ch. 5 - Use curved arrows to show the most likely...Ch. 5 - Construct an explanation for why sulfuric acid is...Ch. 5 - Prob. 16ECh. 5 - Prob. 17ECh. 5 - Prob. 18E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi can you please help me solve this problem? thank youarrow_forwardAn electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.arrow_forwardAt a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forward
- Please do these questions within the SCH4U course please with full steps since I am still unsure how to format my answers! Thank you so much.arrow_forwardWhen two solutions, one of 0.1 M KCl (I) and the other of 0.1 M MCl (II), are brought into contact by a membrane. The cation M cannot cross the membrane. At equilibrium, x moles of K+ will have passed from solution (I) to (II). To maintain the neutrality of the two solutions, x moles of Cl- will also have to pass from I to II. Explain this equality: (0.1 - x)/x = (0.1 + x)/(0.1 - x)arrow_forwardCalculate the variation in the potential of the Pt/MnO4-, Mn2+ pair with pH, indicating the value of the standard potential. Data: E0 = 1.12.arrow_forward
- Given the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt. Calculate the emf of the cell as a function of pH.arrow_forwardThe decimolar calomel electrode has a potential of 0.3335 V at 25°C compared to the standard hydrogen electrode. If the standard reduction potential of Hg22+ is 0.7973 V and the solubility product of Hg2Cl2 is 1.2x 10-18, find the activity of the chlorine ion at this electrode.Data: R = 8.314 J K-1 mol-1, F = 96485 C mol-1, T = 298.15 K.arrow_forward2. Add the following group of numbers using the correct number of significant figures for the answer. Show work to earn full credit such as rounding off the answer to the correct number of significant figures. Replace the question marks with the calculated answers or write the calculated answers near the question marks. 10916.345 37.40832 5.4043 3.94 + 0.0426 ? (7 significant figures)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning 
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY