
Concept explainers
Duffing’s Equation
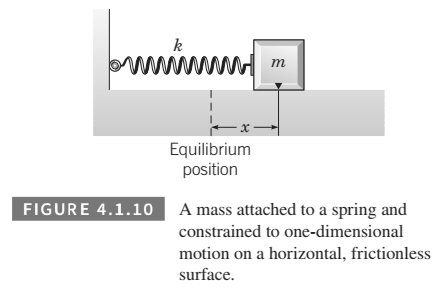
(a) For the spring-mass system shown in Figure
(b) Find the linearized equation for the differential equationderived in part (a) under the assumption that, in its dynamicstate, the maximum displacement of the mass from its equilibrium position is small.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Differential Equations: An Introduction To Modern Methods And Applications 3e Binder Ready Version + Wileyplus Registration Card
Additional Math Textbook Solutions
A First Course in Probability (10th Edition)
Calculus: Early Transcendentals (2nd Edition)
University Calculus: Early Transcendentals (4th Edition)
Calculus for Business, Economics, Life Sciences, and Social Sciences (14th Edition)
Elementary Statistics
Algebra and Trigonometry (6th Edition)
- Each of the following statements is an attempt to show that a given series is convergent or divergent using the Comparison Test (NOT the Limit Comparison Test.) For each statement, enter C (for "correct") if the argument is valid, or enter | (for "incorrect") if any part of the argument is flawed. (Note: if the conclusion is true but the argument that led to it was wrong, you must enter I.) ☐ 1. For all n > 1, seriesΣ In(n) In(n) converges. 2, 1, arctan(n) the series arctan(n) n³ ☐ 4. For all n > 1, 123 converges. 1 n ln(n) series In(n) diverges. 2n . and the seriesΣconverges, so by the Comparison Test, 2, 3, and the series converges, so by the Comparison Test, the series-3 1 converges. ☐ 6. For all n > 2, In(n) >, and the series Σ converges, so by the Comparison Test, the seriesΣ In(n) converges.arrow_forwardIn 2012, the employees of Radcliff Ltd. agreed to purchase 5% of the share capital of 10 million shares of $2 each. There are 20 employees in the plan, and each purchased an equal number of shares. Johnson works at Radcliff Ltd. What would be his ESOP share deduction? $45,000 $25,000 $75,000 $50,000.arrow_forwardInstructions. "I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forward
- Both in images okk. Instructions. "I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forwardQuestion 1: If a barometer were built using oil (p = 0.92 g/cm³) instead of mercury (p = 13.6 g/cm³), would the column of oil be higher than, lower than, or the same as the column of mercury at 1.00 atm? If the level is different, by what factor? Explain. (5 pts) Solution: A barometer works based on the principle that the pressure exerted by the liquid column balances atmospheric pressure. The pressure is given by: P = pgh Since the atmospheric pressure remains constant (P = 1.00 atm), the height of the liquid column is inversely proportional to its density: Step 1: Given Data PHg hol=hgx Poil • Density of mercury: PHg = 13.6 g/cm³ Density of oil: Poil = 0.92 g/cm³ • Standard height of mercury at 1.00 atm: hμg Step 2: Compute Height of Oil = 760 mm = 0.760 m 13.6 hoil = 0.760 x 0.92 hoil = 0.760 × 14.78 hoil = 11.23 m Step 3: Compare Heights Since oil is less dense than mercury, the column of oil must be much taller than that of mercury. The factor by which it is taller is: Final…arrow_forwardQuestion 3: A sealed flask at room temperature contains a mixture of neon (Ne) and nitrogen (N2) gases. Ne has a mass of 3.25 g and exerts a pressure of 48.2 torr. . N2 contributes a pressure of 142 torr. • What is the mass of the N2 in the flask? • Atomic mass of Ne = 20.1797 g/mol • Atomic mass of N = 14.0067 g/mol Solution: We will use the Ideal Gas Law to determine the number of moles of each gas and calculate the mass of N2. PV = nRT where: • P = total pressure • V volume of the flask (same for both gases) n = number of moles of gas • R 0.0821 L atm/mol K • T = Room temperature (assume 298 K) Since both gases are in the same flask, their partial pressures correspond to their mole fractions. Step 1: Convert Pressures to Atmospheres 48.2 PNe = 0.0634 atm 760 142 PN2 = = 0.1868 atm 760 Step 2: Determine Moles of Ne nNe = mass molar mass 3.25 nNe 20.1797 nne 0.1611 mol Step 3: Use Partial Pressure Ratio to Find narrow_forward
- "I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forwardNo chatgpt pls will upvote Already got wrong chatgpt answerarrow_forwardConstruct a table of values for all the nonprincipal Dirichlet characters mod 16.arrow_forward
- MI P X /courses/segura10706/products/171960/pages/611?locale=&platformId=1030&lms=Y ☆ Finish Part I: Mathematics for Elementary and Middle School Teachers Continue in the app JJ 576 Chapter 12. Area of Shapes 9. Determine the area of the shaded shapes in Figure 12.48. Explain your reasoning. 1 unit S Figure 12.48 1 unit unit and the yarn for thearrow_forwardSuppose p > 3 is a prime. Show that (p − 3)!= − P+1 (mod p). Hint: Use Wilson's theorem.arrow_forwardWhich graph represents f(x) = √x-2+3?arrow_forward
 Discrete Mathematics and Its Applications ( 8th I...MathISBN:9781259676512Author:Kenneth H RosenPublisher:McGraw-Hill Education
Discrete Mathematics and Its Applications ( 8th I...MathISBN:9781259676512Author:Kenneth H RosenPublisher:McGraw-Hill Education Mathematics for Elementary Teachers with Activiti...MathISBN:9780134392790Author:Beckmann, SybillaPublisher:PEARSON
Mathematics for Elementary Teachers with Activiti...MathISBN:9780134392790Author:Beckmann, SybillaPublisher:PEARSON
 Thinking Mathematically (7th Edition)MathISBN:9780134683713Author:Robert F. BlitzerPublisher:PEARSON
Thinking Mathematically (7th Edition)MathISBN:9780134683713Author:Robert F. BlitzerPublisher:PEARSON Discrete Mathematics With ApplicationsMathISBN:9781337694193Author:EPP, Susanna S.Publisher:Cengage Learning,
Discrete Mathematics With ApplicationsMathISBN:9781337694193Author:EPP, Susanna S.Publisher:Cengage Learning, Pathways To Math Literacy (looseleaf)MathISBN:9781259985607Author:David Sobecki Professor, Brian A. MercerPublisher:McGraw-Hill Education
Pathways To Math Literacy (looseleaf)MathISBN:9781259985607Author:David Sobecki Professor, Brian A. MercerPublisher:McGraw-Hill Education





