
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
4th Edition
ISBN: 9781260562620
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 75P
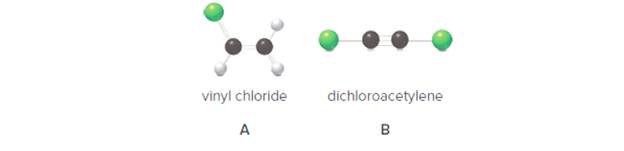
Convert each ball-and-stick model to a Lewis structure and include all nondonded electron pairs. (b) Labell all polar bonds. (c) Is the molecule polar or nonpolar?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw a tripeptide of your choosing at pH 7. Have the N-terminus on the left and the C-terminus on the right. Then:
Draw a triangle around the α-carbons.
Draw a box around the R-groups.
Circle the atoms capable of hydrogen bonding.
Highlight the atoms involved in the formation of the peptide bonds.
What type of structure have you drawn? (primary, secondary, tertiary or quaternary protein structure). make sure its a tripeptide
Don't used hand raiting and don't used Ai solution
Don't used hand raiting and don't used Ai solution
Chapter 4 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
Ch. 4.1 - Use electron-dot symbols to show how a hydrogen...Ch. 4.1 - Use electron-dot symbols to show how two chlorine...Ch. 4.1 - How many covalent bonds are predicted for each...Ch. 4.1 - Fill in the lone pairs on each atom to give every...Ch. 4.1 - Prob. 4.4PCh. 4.2 - Draw a Lewis structure for each covalent molecule....Ch. 4.2 - Draw a Lewis structure for dimethyl ether (C2H6O)...Ch. 4.2 - Prob. 4.4PPCh. 4.2 - Prob. 4.5PCh. 4.2 - Prob. 4.6P
Ch. 4.3 - Prob. 4.7PCh. 4.3 - Prob. 4.8PCh. 4.4 - Prob. 4.5PPCh. 4.4 - Draw resonance structures for each polyatomic...Ch. 4.4 - Nitrous oxide, N2O, is a sweet-smelling gas...Ch. 4.5 - Name each compound: (a) CS2; (b) SO2; (c) PCl5;...Ch. 4.5 - Prob. 4.6PPCh. 4.6 - What is the shape around the indicated atom in...Ch. 4.6 - NaNH2, sodium amid, is a salt that contains a...Ch. 4.6 - Prob. 4.13PCh. 4.7 - Using the trends in the periodic table, rank the...Ch. 4.7 - Use electronegativity values to classify the...Ch. 4.8 - Label the polar bonds in each molecule, and then...Ch. 4.9 - Label the polar bonds in each molecule, and then...Ch. 4.9 - Use the ball-and-stick model of dihydrocapsaicin...Ch. 4 - For each pair of compounds, classify the bonding...Ch. 4 - For each pair of compounds, classify the bonding...Ch. 4 - Prob. 17PCh. 4 - How many bonds and lone pairs are typically...Ch. 4 - Prob. 19PCh. 4 - Fill in the lone pairs needed to give the main...Ch. 4 - Prob. 21PCh. 4 - Convert the 3-D model of the general anesthetic...Ch. 4 - Draw a valid Lewis structure for each molecule. Hl...Ch. 4 - Draw a valid Lewis structure for each molecule....Ch. 4 - Prob. 25PCh. 4 - Prob. 26PCh. 4 - Draw a valid Lewis structure for...Ch. 4 - Draw a valid Lewis structure for phosgene, CCl2O ,...Ch. 4 - Draw a valid Lewis structure for each ion: (a)...Ch. 4 - Draw a valid Lewis structure for each ion: (a)...Ch. 4 - Prob. 31PCh. 4 - Keeping in mind that some elements violate the...Ch. 4 - Prob. 33PCh. 4 - Prob. 34PCh. 4 - Prob. 35PCh. 4 - Prob. 36PCh. 4 - Prob. 37PCh. 4 - Label each pair of compounds are resonance...Ch. 4 - Prob. 39PCh. 4 - Draw three resonance structures for the nitrate...Ch. 4 - Name each covalent compound. PBr3 SO3 NCl3 P2S5Ch. 4 - Name each covalent compound. SF6 CBr4 N2O P4O10Ch. 4 - Prob. 43PCh. 4 - Prob. 44PCh. 4 - Add lone pairs where needed to give octets and...Ch. 4 - Add lone pairs where needed to give octets and...Ch. 4 - Prob. 47PCh. 4 - Match each compound with one of the molecular...Ch. 4 - Prob. 49PCh. 4 - Add lone pairs where needed to give octets and...Ch. 4 - Prob. 51PCh. 4 - Considering each of the given ball-and stick...Ch. 4 - Prob. 53PCh. 4 - Prob. 54PCh. 4 - Prob. 55PCh. 4 - Predict the bond angles around the indicated atoms...Ch. 4 - Prob. 57PCh. 4 - Prob. 58PCh. 4 - Rank the atoms in each group in order of...Ch. 4 - Prob. 60PCh. 4 - Prob. 61PCh. 4 - Identify elements D, E, and F and rank them in...Ch. 4 - Prob. 63PCh. 4 - Using electronegativity values, classify the bond...Ch. 4 - Label the bond formed between carbon and each of...Ch. 4 - Label the bond formed between fluroine and each of...Ch. 4 - Which bond in each pair is more polar-that is, has...Ch. 4 - Which bond in each pair is more polar-that is, has...Ch. 4 - Prob. 69PCh. 4 - Prob. 70PCh. 4 - Label the polar bonds and then decide if each...Ch. 4 - Label the polar bonds and then decide if each...Ch. 4 - Prob. 73PCh. 4 - Explain why H2O is a polar molecule but H2S is...Ch. 4 - Convert each ball-and-stick model to a Lewis...Ch. 4 - Convert each ball-and-stick model to a Lewis...Ch. 4 - Answer the following questions about the molecule...Ch. 4 - Answer the following question about the molecule...Ch. 4 - Prob. 79PCh. 4 - Lactic acid gives sour milk its distinctive taste....Ch. 4 - Use the ball-and-stick model of zingerone, a...Ch. 4 - Prob. 82PCh. 4 - Prob. 83PCh. 4 - Prob. 84PCh. 4 - Isobutyl cyanoacrylate is used in medical glues to...Ch. 4 - Prob. 86PCh. 4 - Cyclopropane is a stable compound that contains...Ch. 4 - Prob. 88CPCh. 4 - Prob. 89CPCh. 4 - Prob. 90CP
Additional Science Textbook Solutions
Find more solutions based on key concepts
The validity of a scientific law.
Physical Universe
Give the IUPAC name for each compound.
Organic Chemistry
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
11. In the early 1800s, French naturalist Jean Baptiste Lamarck suggested that the best explanation for the rel...
Campbell Biology: Concepts & Connections (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used Ai solution and don't used hand raitingarrow_forward> Organic Functional Groups Naming and drawing alkyl halides structure CI Br CI CI Explanation Check 2 name 1-chloro-2,4,9-trimethylnonane CI 2-iodo-2,3-dimethylbutane FEB 19 € E M tv MacBook Airarrow_forwardCan you please explain to me this problem im very confused and lost. Help me step by step and in detail im soo lost.arrow_forward
- 2) There are many forms of cancer, all of which involve abnormal cell growth. The growth and production of cells, called cell proliferation, is known to involve an enzyme called protein farnesyltransferase (PFTase). It is thought that inhibitors pf PFTase may be useful as anticancer drugs. The following molecule showed moderate activity as a potential PFTase inhibitor. Draw all stereoisomers of this compound. HO OHarrow_forwardConsidering rotation around the bond highlighted in red, draw the Newman projection for the most stable and least stable conformations when viewed down the red bond in the direction of the arrow. Part 1 of 2 H₁₂C H H Draw the Newman projection for the most stable conformation. Select a template to begin. Part 2 of 2 Draw the Newman projection for the least stable conformation. G 心arrow_forwardpersonality of each of them in terms of nucleophile vs. electrophile (some can be considered acids/bases but we are not looking at that here). Note you may have to use your growing intuition to figure out the personality of one of the molecules below but I believe in you! Rationalize it out based on what we have called strong versus weak electrophiles in past mechanisms. Consider using the memes below to help guide your understanding! A OH O B CH3 C Molecule A: [Select] Molecule B: [Select] Molecule C: [Select] Molecule D: [Select] > H D OHarrow_forward
- 4) Which oxygen atom in the structure below is most basic / nucleophilic? Please explain by discussing the electron density around each oxygen atom. Show at least three resonance structures for the compound. оогоarrow_forwardCan you show me this problem. Turn them into lewis dot structures for me please and then answer the question because I cant seem to comprehend it/ The diagrams on the picture look too small I guess.arrow_forwardThe fire releases 2.80 x 107 Joules of heat energy for each liter of oil burned. The water starts out at 24.5 °C, raising the water's temperature up to 100 °C, and then raises the temperature of the resulting steam up to 325 °C. How many liters of water will be needed to absorb the heat from the fire in this way, for each 1.0 liter of crude oil burned? 4186 J/(kg°C) = heat of water 2020 J/(kg°C) = heat of steam 2,256,000 (i.e. 2.256 x 106) J/kg = latent heat of vaporization for water (at the boiling point of 100 °C).arrow_forward
- 6 Which of the following are likely to be significant resonance structures of a resonance hybrid? Draw another resonance structure for each of the compounds you select as being a resonance form. (A Br: Br: A B C D Earrow_forwardWrite the systematic (IUPAC) name for the following organic molecules. Note for advanced students: you do not need to include any E or Z prefixes in your names. Br structure Br Br Oweuarrow_forwardConservation of mass was discussed in the background. Describe how conservation of mass (actual, not theoretical) could be checked in the experiment performed.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY