
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
4th Edition
ISBN: 9781260562620
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 38P
Label each pair of compounds are resonance structures or not resonance structures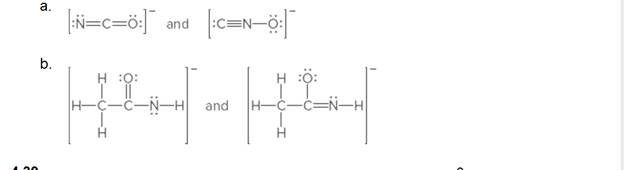
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(c) The following data have been obtained for the hydrolysis of sucrose, C12H22O11, to
glucose, C6H12O6, and fructose C6H12O6, in acidic solution:
C12H22O11 + H2O → C6H12O6 + C6H12O6
[sucrose]/mol dm³
t/min
0
0.316
14
0.300
39
0.274
60
0.256
80
0.238
110
0.211
(i) Graphically prove the order of the reaction and determine the rate constant of the
reaction.
(ii) Determine the half-life, t½ for the hydrolysis of sucrose.
(III) adsorbent
(b) Adsorption of the hexacyanoferrate (III) ion, [Fe(CN)6] ³, on y-Al2O3 from aqueous
solution was examined. The adsorption was modelled using a modified Langmuir
isotherm, yielding the following values of Kat pH = 6.5:
(ii)
T/K
10-10 K
280
2.505
295
1.819
310
1.364
325
1.050
Determine the enthalpy of adsorption, AadsHⓇ.
If the reported value of entropy of adsorption, Aads Se = 146 J K-1 mol-1 under the above
conditions, determine Aads Gº.
with full details solution please
Chapter 4 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
Ch. 4.1 - Use electron-dot symbols to show how a hydrogen...Ch. 4.1 - Use electron-dot symbols to show how two chlorine...Ch. 4.1 - How many covalent bonds are predicted for each...Ch. 4.1 - Fill in the lone pairs on each atom to give every...Ch. 4.1 - Prob. 4.4PCh. 4.2 - Draw a Lewis structure for each covalent molecule....Ch. 4.2 - Draw a Lewis structure for dimethyl ether (C2H6O)...Ch. 4.2 - Prob. 4.4PPCh. 4.2 - Prob. 4.5PCh. 4.2 - Prob. 4.6P
Ch. 4.3 - Prob. 4.7PCh. 4.3 - Prob. 4.8PCh. 4.4 - Prob. 4.5PPCh. 4.4 - Draw resonance structures for each polyatomic...Ch. 4.4 - Nitrous oxide, N2O, is a sweet-smelling gas...Ch. 4.5 - Name each compound: (a) CS2; (b) SO2; (c) PCl5;...Ch. 4.5 - Prob. 4.6PPCh. 4.6 - What is the shape around the indicated atom in...Ch. 4.6 - NaNH2, sodium amid, is a salt that contains a...Ch. 4.6 - Prob. 4.13PCh. 4.7 - Using the trends in the periodic table, rank the...Ch. 4.7 - Use electronegativity values to classify the...Ch. 4.8 - Label the polar bonds in each molecule, and then...Ch. 4.9 - Label the polar bonds in each molecule, and then...Ch. 4.9 - Use the ball-and-stick model of dihydrocapsaicin...Ch. 4 - For each pair of compounds, classify the bonding...Ch. 4 - For each pair of compounds, classify the bonding...Ch. 4 - Prob. 17PCh. 4 - How many bonds and lone pairs are typically...Ch. 4 - Prob. 19PCh. 4 - Fill in the lone pairs needed to give the main...Ch. 4 - Prob. 21PCh. 4 - Convert the 3-D model of the general anesthetic...Ch. 4 - Draw a valid Lewis structure for each molecule. Hl...Ch. 4 - Draw a valid Lewis structure for each molecule....Ch. 4 - Prob. 25PCh. 4 - Prob. 26PCh. 4 - Draw a valid Lewis structure for...Ch. 4 - Draw a valid Lewis structure for phosgene, CCl2O ,...Ch. 4 - Draw a valid Lewis structure for each ion: (a)...Ch. 4 - Draw a valid Lewis structure for each ion: (a)...Ch. 4 - Prob. 31PCh. 4 - Keeping in mind that some elements violate the...Ch. 4 - Prob. 33PCh. 4 - Prob. 34PCh. 4 - Prob. 35PCh. 4 - Prob. 36PCh. 4 - Prob. 37PCh. 4 - Label each pair of compounds are resonance...Ch. 4 - Prob. 39PCh. 4 - Draw three resonance structures for the nitrate...Ch. 4 - Name each covalent compound. PBr3 SO3 NCl3 P2S5Ch. 4 - Name each covalent compound. SF6 CBr4 N2O P4O10Ch. 4 - Prob. 43PCh. 4 - Prob. 44PCh. 4 - Add lone pairs where needed to give octets and...Ch. 4 - Add lone pairs where needed to give octets and...Ch. 4 - Prob. 47PCh. 4 - Match each compound with one of the molecular...Ch. 4 - Prob. 49PCh. 4 - Add lone pairs where needed to give octets and...Ch. 4 - Prob. 51PCh. 4 - Considering each of the given ball-and stick...Ch. 4 - Prob. 53PCh. 4 - Prob. 54PCh. 4 - Prob. 55PCh. 4 - Predict the bond angles around the indicated atoms...Ch. 4 - Prob. 57PCh. 4 - Prob. 58PCh. 4 - Rank the atoms in each group in order of...Ch. 4 - Prob. 60PCh. 4 - Prob. 61PCh. 4 - Identify elements D, E, and F and rank them in...Ch. 4 - Prob. 63PCh. 4 - Using electronegativity values, classify the bond...Ch. 4 - Label the bond formed between carbon and each of...Ch. 4 - Label the bond formed between fluroine and each of...Ch. 4 - Which bond in each pair is more polar-that is, has...Ch. 4 - Which bond in each pair is more polar-that is, has...Ch. 4 - Prob. 69PCh. 4 - Prob. 70PCh. 4 - Label the polar bonds and then decide if each...Ch. 4 - Label the polar bonds and then decide if each...Ch. 4 - Prob. 73PCh. 4 - Explain why H2O is a polar molecule but H2S is...Ch. 4 - Convert each ball-and-stick model to a Lewis...Ch. 4 - Convert each ball-and-stick model to a Lewis...Ch. 4 - Answer the following questions about the molecule...Ch. 4 - Answer the following question about the molecule...Ch. 4 - Prob. 79PCh. 4 - Lactic acid gives sour milk its distinctive taste....Ch. 4 - Use the ball-and-stick model of zingerone, a...Ch. 4 - Prob. 82PCh. 4 - Prob. 83PCh. 4 - Prob. 84PCh. 4 - Isobutyl cyanoacrylate is used in medical glues to...Ch. 4 - Prob. 86PCh. 4 - Cyclopropane is a stable compound that contains...Ch. 4 - Prob. 88CPCh. 4 - Prob. 89CPCh. 4 - Prob. 90CP
Additional Science Textbook Solutions
Find more solutions based on key concepts
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Zeroth Order Reaction In a certain experiment the decomposition of hydrogen iodide on finely divided gold is zeroth order with respect to HI. 2HI(g) Au H2(g) + 12(9) Rate = -d[HI]/dt k = 2.00x104 mol L-1 s-1 If the experiment has an initial HI concentration of 0.460 mol/L, what is the concentration of HI after 28.0 minutes? 1 pts Submit Answer Tries 0/5 How long will it take for all of the HI to decompose? 1 pts Submit Answer Tries 0/5 What is the rate of formation of H2 16.0 minutes after the reaction is initiated? 1 pts Submit Answer Tries 0/5arrow_forwardangelarodriguezmunoz149@gmail.com Hi i need help with this question i am not sure what the right answers are.arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Don't used hand raitingarrow_forwardDon't used Ai solutionarrow_forwardSaved v Question: I've done both of the graphs and generated an equation from excel, I just need help explaining A-B. Below is just the information I used to get the graphs obtain the graph please help. Prepare two graphs, the first with the percent transmission on the vertical axis and concentration on the horizontal axis and the second with absorption on the vertical axis and concentration on the horizontal axis. Solution # Unknown Concentration (mol/L) Transmittance Absorption 9.88x101 635 0.17 1.98x101 47% 0.33 2.95x101 31% 0.51 3.95x10 21% 0.68 4.94x10 14% 24% 0.85 0.62 A.) Give an equation that relates either the % transmission or the absorption to the concentration. Explain how you arrived at your equation. B.) What is the relationship between the percent transmission and the absorption? C.) Determine the concentration of the ironlll) salicylate in the unknown directly from the graph and from the best fit trend-line (least squares analysis) of the graph that yielded a straight…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning 
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY