
(a)
Interpretation:
The number of elements that forms a negatively charged ions among the following highlighted elements in the below periodic table has to be identified.
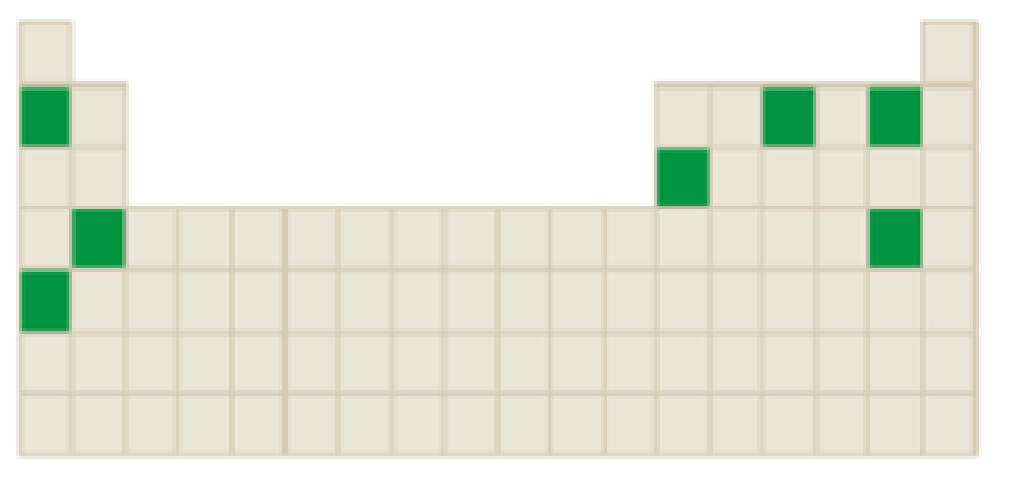
Concept Introduction:
Atoms tend to gain or lose electrons until they obtain an electron configuration that is the same as that of a noble gas.
The neutral atom has equal number of protons and electrons. Gaining or loosing of a electrons of an atoms form ions.
Atoms form their ions to attain noble gas configuration.
- Group IA, IIA and IIIA are metal atoms containing one, two or three valence electrons. These metal atoms lose their valence electrons to get noble gas configuration.
- Group VA, VIA VIIA are non-metal atoms containing five, six or seven valence electrons. These non-metal atoms acquire electrons to get noble gas configuration.
- Group IVA group elements have four valence electrons. These elements either gain or lose their electrons to get noble gas configuration.
(b)
Interpretation:
The elements that forms ions through gain of electrons among the following highlighted elements in the below periodic table has to be identified.
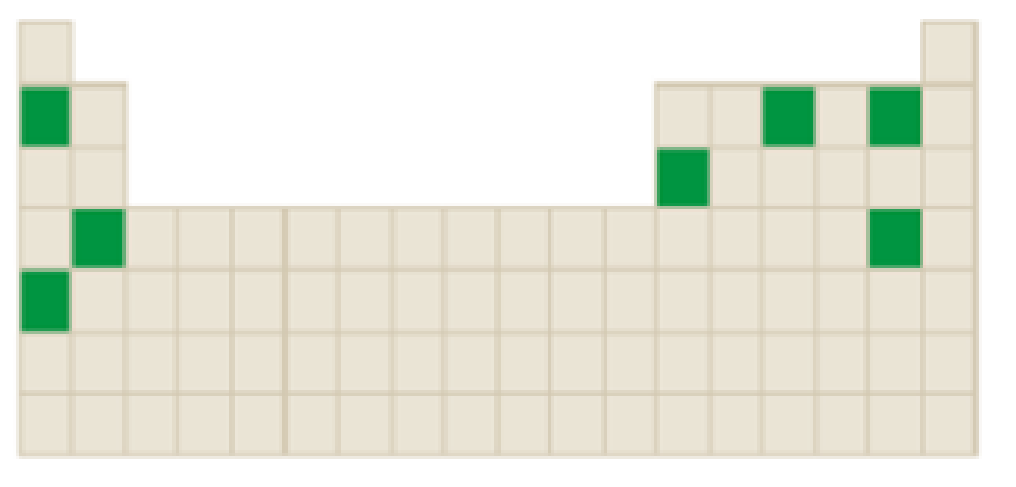
Concept Introduction:
Atoms tend to gain or lose electrons until they obtain an electron configuration that is the same as that of a noble gas.
The neutral atom has equal number of protons and electrons. Gaining or loosing of a electrons of an atoms form ions.
Atoms form their ions to attain noble gas configuration.
- Group IA, IIA and IIIA are metal atoms containing one, two or three valence electrons. These metal atoms lose their valence electrons to get noble gas configuration.
- Group VA, VIA VIIA are non-metal atoms containing five, six or seven valence electrons. These non-metal atoms acquire electrons to get noble gas configuration.
- Group IVA group elements have four valence electrons. These elements either gain or lose their electrons to get noble gas configuration.
(c)
Interpretation:
The elements which forms ions that has a charge magnitude of
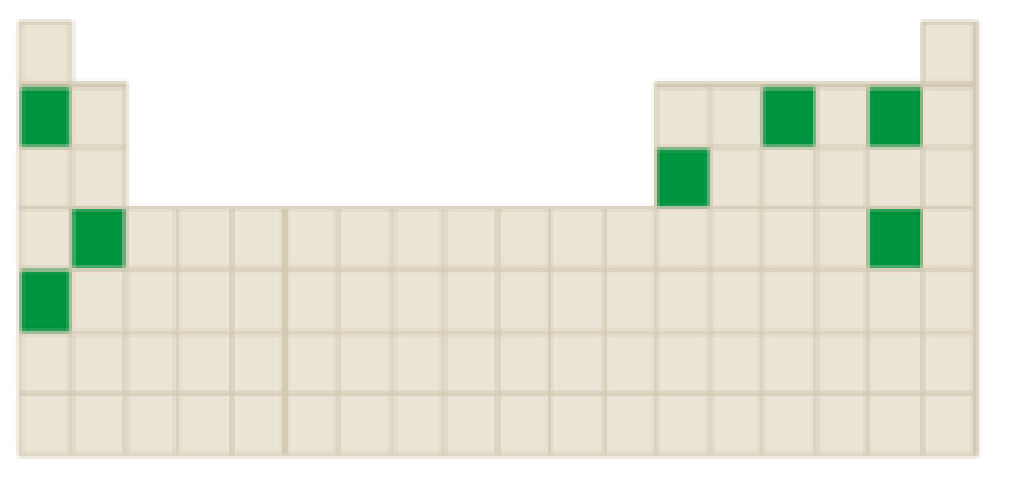
Concept Introduction:
Atoms tend to gain or lose electrons until they obtain an electron configuration that is the same as that of a noble gas.
The neutral atom has equal number of protons and electrons. Gaining or loosing of a electrons of an atoms form ions.
Atoms form their ions to attain noble gas configuration.
- Group IA, IIA and IIIA are metal atoms containing one, two or three valence electrons. These metal atoms lose their valence electrons to get noble gas configuration.
- Group VA, VIA VIIA are non-metal atoms containing five, six or seven valence electrons. These non-metal atoms acquire electrons to get noble gas configuration.
- Group IVA group elements have four valence electrons. These elements either gain or lose their electrons to get noble gas configuration.
(d)
Interpretation:
The elements that forms an ion that involves loss of two or more electrons among the following highlighted elements in the below periodic table has to be identified.
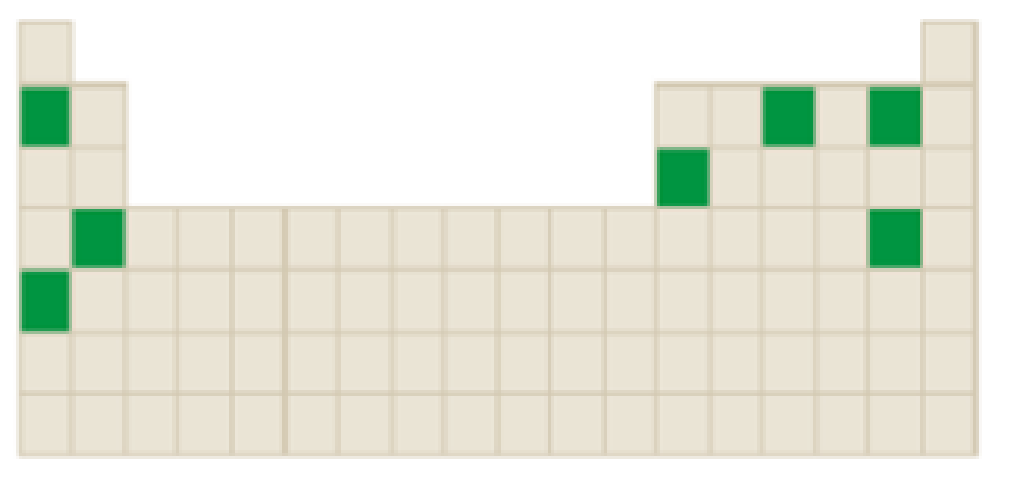
Concept Introduction:
Atoms tend to gain or lose electrons until they obtain an electron configuration that is the same as that of a noble gas.
The neutral atom has equal number of protons and electrons. Gaining or loosing of a electrons of an atoms form ions.
Atoms form their ions to attain noble gas configuration.
- Group IA, IIA and IIIA are metal atoms containing one, two or three valence electrons. These metal atoms lose their valence electrons to get noble gas configuration.
- Group VA, VIA VIIA are non-metal atoms containing five, six or seven valence electrons. These non-metal atoms acquire electrons to get noble gas configuration.
- Group IVA group elements have four valence electrons. These elements either gain or lose their electrons to get noble gas configuration.
Trending nowThis is a popular solution!

Chapter 4 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry [2 ONLY]. A -CH2COOH 1. LIAIH THF, heat 2 HO B. C. CH₂Br Br 1. NaCN, acetone 2 H₂O, heat 1. Mg ether 3 HO Z CO₂arrow_forwardWhat is the order of increasing acidity for the following compounds? (least to most) [2 ONLY] A. COOH COOH COOH COOH 6666 a. IV, I, III, II b. III, II, I, IV с. II, III, I, IV d. III, II, IV, I slingoros CH3 IV woled noise bizarrow_forwardWith this, please answer the following questions: 1.) draw the structure of the electrophilic intermediate in this reaction. 2.) what is the role of the AlCl3 in the reaction 3.) write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and include all intermediate structuresarrow_forward
- Consider the data below to answer the following questions. Cyanohydrins are important intermediates in the synthesis of a-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. Unfortunately, when a cyanohydrin is treated with aqueous base the original carbonyl compound is isolated. HO H HCEN H-3- HO' NaOH HO cyanohychin a. a nucleophilic substitution b. an electrophilic addition C10 OH CH-COOH A. The reaction of an aldehyde with hydrogen cyanide is an example of + NaCN + H₂O reaction. H- C. an electrophilic substitution d. a nucleophilic addition B. Identify the electrophile in the reaction of benzaldehyde with hydrogen cyanide.arrow_forwardRefer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A. Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardGive the major organic product(s) of each of the following reactions or sequences of reactions. Show all relevant stereochemistry.[4 only] CH3 A. B. HNO H₂Pt H₂SO4 hano NaN 1. LIAH ether Br 4 2 H₂O C. D. E. CH3CH2-CH2CH3 + HCl Br NH₂ CH3 ON CH-CH3 Br HNOZ CUCI 11,504 HC) 1. HNO H SO NH₂ 2 UMarrow_forward
- Consider the Grignard reaction below to answer the following questions. A Mgar 1. ether + MyC CH3 2H3O C B a. The electrophile in this reaction is: b. The nucleophile in this reaction is: c. The alcohol product can be classified as a: a. 1° alcohol b. 2° alcohol C. 3° alcohol d. 4° alcohol HO CH3 CHarrow_forwardGive the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry A. CH₂OH PCC CH2Cl2 HOO B. H KCN HCN of b C. 1. CH,MgBr, ether 2 HO* D. Choose the BEST reagent for carrying out each of the following conversions. CO₂CH3 CO₂CH3 OH CO₂H сон ن نے a. LiAlH4, ether at abinayo iss c b. NaBH4, ethanol C. CrO3, pyridine d. H₂/Pd d notsiolarrow_forwardChoose the best reagent for carrying out the following reactions from the list below. Place the letter of the reagent(s) in the box over the reaction arrow. Use only one letter per box. OH OH CH CH CH3 CHS CH3 f OH OCH 3 H A. NaH, then CHI B. C. m-ClC6H4COзH D. E. warm H2SO4/H₂O F. G. H₂/Pd H. I. Cl₂, H₂O J. NaOCH3, CH3OH CH3MgBr in ether, then H3O+ Hg(O2CCF3)2, CH3OH PCC, CH2Cl2 LiAlH4 in ether, then H3O+arrow_forward
- What is the product of the reaction of 2,4-pentanedione with phenylhydrazine?arrow_forwardIn the reaction of naphthalene with CrO3 in acetic acid. Indicate whether a different product is obtained if carried out at 25°C or with heating (A).arrow_forwardQUESTION: Fill in the answers in the empty green boxes 1. Step 2 2. Step 3 3. Step 4 (SUM) 4. Step 5 (df) (GIVEN) 5. Determine S y/x value *The data values have been provided in the worksheet attached in the first image*arrow_forward

 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





