
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
7th Edition
ISBN: 9781305081086
Author: STOKER, H. Stephen
Publisher: Brooks Cole
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.27EP
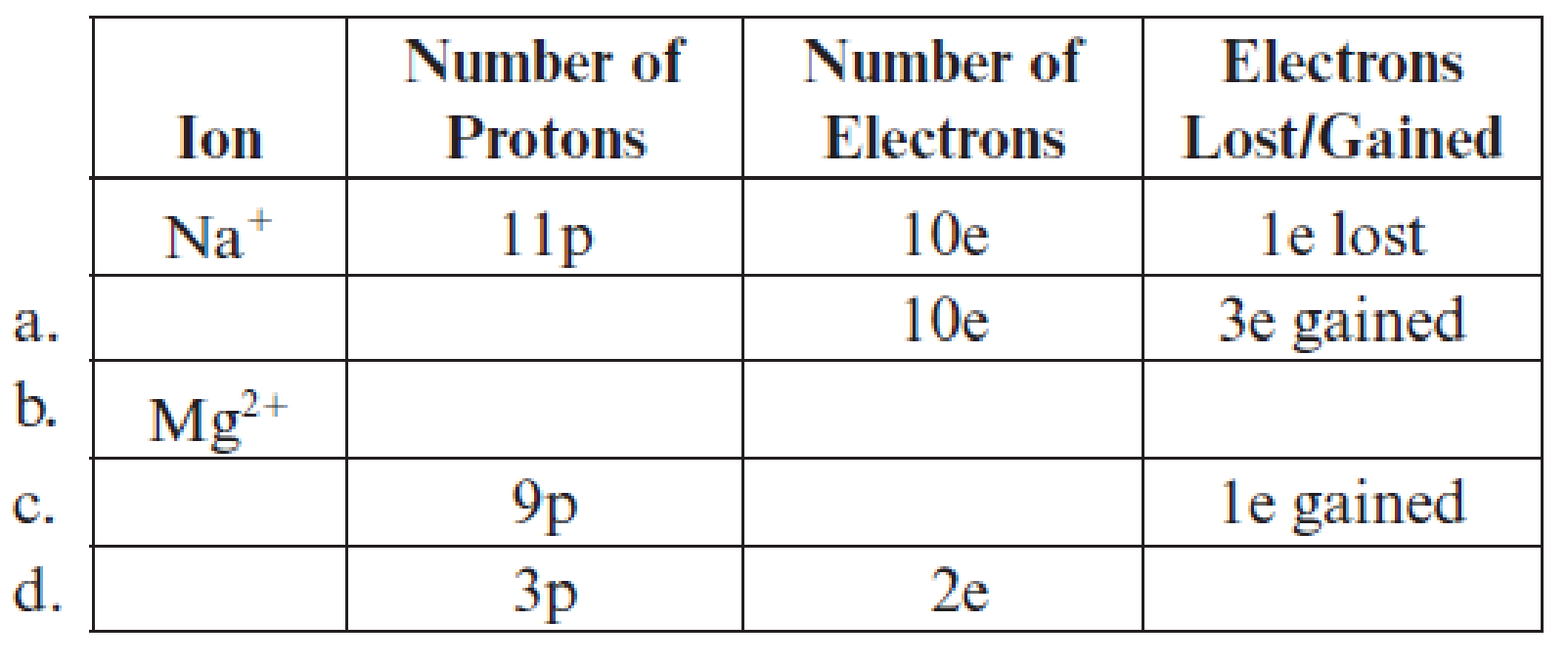
Fill in the blanks in each line in the following table. The first line is already completed as an example.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
T
1
2
4
5
3
6
7
9
8
10
11
CHEMISTRY
Writing Chemical Formulae for Ionic Compounds with Polyatomic Ions
Write the correct chemical formulae for the ionic compound using the monoatomic ions.
Chemical formulae are written as
● Metal (cation) first, Nonmetal (anion) second.
Use subscripts to the right of the chemical symbol for an element if there is more
than one present in the molecule,
All molecules must be neutral: Positives = Negatives
• Use the charts for the monoatomic cations and anions, and polyatomic ions to help
you
Elements
K & NO3
K & SO4
Ca & OH
Ca & CO3
Al & OH
Al & CIO4
NH4 & SO4
NH4 &CIO3
Mg & HCO3
12 Mg & MnO4
Na & HCO3
Na & PO4
Chemical Formula
13
14
15
16
17
18
19
20
21
22
23
24
Chemical Formula
Elements
Fe(II) & SO4
Fe(III) & OH
Cu(I) & CN
Cu(I) & CO3
Cu(I) & NO3
Mn(II) & CIO4
Ti(IV) & SCN
Ti(IV) & CrO4
Sc(III) & PO4
Sc(III) & CN
Pb(IV) & MnO4
Pb(II) & CO3
14.
A A typical soap molecule is made up of a
polyatomic anion associated with a cation. The
polyatomic anion contains hydrogen, carbon, and
oxygen. One soap molecule has 18 carbon atoms and
contains 70.5% carbon, 11.5% hydrogen, and 10.4%
oxygen by mass. It also contains one alkali metal ion.
Identify this alkali metal ion.
(13:
od
ib
Chapter 6 Proportions in Chemical Compounds MHR 267
●
The hydroxides formed by three elements of period 3 are given in the following table.
Write the chemical formulas for the hydroxides formed by the corresponding elements in period 4 for groups 1A, 2A, and 3A.
Express your answers as chemical formulas separated by commas.
Period
Group 1A
Group 2A
Group 3A
period 3
NaOH
Mg(OH)2
Al(OH)3
period 4
?
?
?
Chapter 4 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
Ch. 4.1 - Prob. 1QQCh. 4.1 - Prob. 2QQCh. 4.1 - Prob. 3QQCh. 4.2 - How many valence electrons are present in an atom...Ch. 4.2 - Prob. 2QQCh. 4.2 - Prob. 3QQCh. 4.2 - Prob. 4QQCh. 4.2 - Which of the following elements would have the...Ch. 4.3 - Prob. 1QQCh. 4.3 - Prob. 2QQ
Ch. 4.3 - Prob. 3QQCh. 4.4 - In terms of subatomic particles, a Ca2+ ion...Ch. 4.4 - Prob. 2QQCh. 4.4 - Prob. 3QQCh. 4.4 - Prob. 4QQCh. 4.5 - An atom with a 1s22s22p4 electron configuration...Ch. 4.5 - Prob. 2QQCh. 4.5 - Prob. 3QQCh. 4.5 - Prob. 4QQCh. 4.5 - Prob. 5QQCh. 4.6 - Prob. 1QQCh. 4.6 - Prob. 2QQCh. 4.6 - Prob. 3QQCh. 4.7 - What is the chemical formula of the ionic compound...Ch. 4.7 - What is the chemical formula of the ionic compound...Ch. 4.7 - Given that Z2 ions are present in the ionic...Ch. 4.7 - Prob. 4QQCh. 4.8 - Prob. 1QQCh. 4.8 - Which of the following is a correct description of...Ch. 4.9 - Prob. 1QQCh. 4.9 - Prob. 2QQCh. 4.9 - Prob. 3QQCh. 4.9 - Prob. 4QQCh. 4.9 - Prob. 5QQCh. 4.9 - Prob. 6QQCh. 4.9 - The correct name for the binary ionic compound...Ch. 4.9 - Prob. 8QQCh. 4.10 - Prob. 1QQCh. 4.10 - Which of the following statements about polyatomic...Ch. 4.10 - The nitrate, sulfate, and phosphate ions have,...Ch. 4.10 - Prob. 4QQCh. 4.11 - Prob. 1QQCh. 4.11 - Prob. 2QQCh. 4.11 - Prob. 3QQCh. 4.11 - Prob. 4QQCh. 4.11 - Prob. 5QQCh. 4.11 - What is the chemical formula for the compound...Ch. 4 - Contrast the two general types of chemical bonds...Ch. 4 - Contrast the two general types of chemical...Ch. 4 - How many valence electrons do atoms with the...Ch. 4 - How many valence electrons do atoms with the...Ch. 4 - Prob. 4.5EPCh. 4 - Prob. 4.6EPCh. 4 - Write the complete electron configuration for each...Ch. 4 - Write the complete electron configuration for each...Ch. 4 - Prob. 4.9EPCh. 4 - For each of the following pairs of representative...Ch. 4 - How many of the highlighted elements in the...Ch. 4 - How many of the highlighted elements in the...Ch. 4 - Draw Lewis symbols for atoms of each of the...Ch. 4 - Draw Lewis symbols for atoms of each of the...Ch. 4 - Each of the following Lewis symbols represents a...Ch. 4 - Each of the following Lewis symbols represents a...Ch. 4 - Prob. 4.17EPCh. 4 - Prob. 4.18EPCh. 4 - What is the chemical property of the noble gases...Ch. 4 - Prob. 4.20EPCh. 4 - Prob. 4.21EPCh. 4 - Prob. 4.22EPCh. 4 - Give the chemical symbol for each of the following...Ch. 4 - Give the chemical symbol for each of the following...Ch. 4 - What would be the chemical symbol for an ion with...Ch. 4 - What would be the chemical symbol for an ion with...Ch. 4 - Fill in the blanks in each line in the following...Ch. 4 - Fill in the blanks in each line in the following...Ch. 4 - Fill in the blanks in each line of the following...Ch. 4 - Fill in the blanks in each line of the following...Ch. 4 - Identify element X by giving its chemical symbol,...Ch. 4 - Prob. 4.32EPCh. 4 - Prob. 4.33EPCh. 4 - Prob. 4.34EPCh. 4 - Prob. 4.35EPCh. 4 - Draw Lewis symbols for the following ions. a. O2...Ch. 4 - What is the charge on the monatomic ion formed by...Ch. 4 - What is the charge on the monatomic ion formed by...Ch. 4 - Indicate the number of electrons lost or gained...Ch. 4 - Indicate the number of electrons lost or gained...Ch. 4 - Which noble gas has an electron configuration...Ch. 4 - Prob. 4.42EPCh. 4 - Which noble gas is isoelectronic with each of the...Ch. 4 - Which noble gas is isoelectronic with each of the...Ch. 4 - Prob. 4.45EPCh. 4 - Indicate whether or not each of the following...Ch. 4 - Prob. 4.47EPCh. 4 - Prob. 4.48EPCh. 4 - Prob. 4.49EPCh. 4 - Write the electron configuration of the following....Ch. 4 - How many valence electrons are present in each of...Ch. 4 - Prob. 4.52EPCh. 4 - Using Lewis structures, show how ionic compounds...Ch. 4 - Using Lewis structures, show how ionic compounds...Ch. 4 - The following Lewis symbols for ions have the...Ch. 4 - Prob. 4.56EPCh. 4 - Prob. 4.57EPCh. 4 - Prob. 4.58EPCh. 4 - Prob. 4.59EPCh. 4 - Prob. 4.60EPCh. 4 - The component elements for four binary ionic...Ch. 4 - Prob. 4.62EPCh. 4 - Write the complete chemical formula (symbol and...Ch. 4 - Write the complete chemical formula (symbol and...Ch. 4 - Write the chemical formula for the ionic compound...Ch. 4 - Prob. 4.66EPCh. 4 - Prob. 4.67EPCh. 4 - What is the chemical formula of the ionic compound...Ch. 4 - A representative element (X) forms an ion with a 2...Ch. 4 - A representative element (Z) forms an ion with a...Ch. 4 - Prob. 4.71EPCh. 4 - The following questions pertain to the ionic...Ch. 4 - Prob. 4.73EPCh. 4 - Prob. 4.74EPCh. 4 - Prob. 4.75EPCh. 4 - Prob. 4.76EPCh. 4 - Prob. 4.77EPCh. 4 - Prob. 4.78EPCh. 4 - Prob. 4.79EPCh. 4 - Which of the following binary compounds would be...Ch. 4 - Name the following binary ionic compounds, each of...Ch. 4 - Name the following binary ionic compounds, each of...Ch. 4 - Calculate the charge on the metal ion in the...Ch. 4 - Calculate the charge on the metal ion in the...Ch. 4 - Prob. 4.85EPCh. 4 - Prob. 4.86EPCh. 4 - Prob. 4.87EPCh. 4 - Prob. 4.88EPCh. 4 - Name each of the following binary ionic compounds....Ch. 4 - Name each of the following binary ionic compounds....Ch. 4 - Prob. 4.91EPCh. 4 - Name each compound in the following pairs of...Ch. 4 - Prob. 4.93EPCh. 4 - Write chemical formulas for the following binary...Ch. 4 - Prob. 4.95EPCh. 4 - Write chemical formulas for the following binary...Ch. 4 - Prob. 4.97EPCh. 4 - Prob. 4.98EPCh. 4 - Fill in the blanks in each line of the following...Ch. 4 - Prob. 4.100EPCh. 4 - Prob. 4.101EPCh. 4 - Prob. 4.102EPCh. 4 - Prob. 4.103EPCh. 4 - How many oxygen atoms are present in each of the...Ch. 4 - Prob. 4.105EPCh. 4 - Prob. 4.106EPCh. 4 - Prob. 4.107EPCh. 4 - Prob. 4.108EPCh. 4 - Prob. 4.109EPCh. 4 - Prob. 4.110EPCh. 4 - How many ions are present per formula unit in each...Ch. 4 - Prob. 4.112EPCh. 4 - Name the following compounds, all of which contain...Ch. 4 - Prob. 4.114EPCh. 4 - Prob. 4.115EPCh. 4 - Prob. 4.116EPCh. 4 - Prob. 4.117EPCh. 4 - Write formulas for the following compounds, all of...Ch. 4 - Write chemical formulas for the following...Ch. 4 - Write chemical formulas for the following...Ch. 4 - Prob. 4.121EPCh. 4 - Prob. 4.122EPCh. 4 - Prob. 4.123EPCh. 4 - Prob. 4.124EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Students conducted an experiment to observe the properties of four elements. The results of their observations are in the table below. Based on the information in the table, Sample 3 would likely be classified as a(n) — alkali metal halogen noble gas transition metalarrow_forwardThe hydroxides formed by three elements of period 3 are given in the following table. Period Group 1A Group 2A Group 3A Period 3 NaOHNaOH Mg(OH)2Mg(OH)2 Al(OH)3Al(OH)3 Period 4 ? ? ? Write the chemical formulas for the hydroxides formed by the corresponding elements in period 4 for groups 1A, 2A, and 3A.arrow_forwardif a neutral element has this chemical symbol, how many electrons does it have?arrow_forward
- 1. The Group 18/VIII elements are known as the noble gases. Answer the following true or false questions about them. all are nonmetals they like to gain electrons they like to form ions they don't react with other ions or elements 2. The Group 17/VII elements are known as the halogens. Answer the following true or false questions about them. all are nonmetals they like to form anions when they gain the electron they are attracted to cationsarrow_forwardWhat is the chemical formula of the salt formed between Sodium and Sulfur? What is the name of the salt formed between Sodium and Sulfur? What is the chemical formula of the salt formed between Magnesium and Bromine? What is the name of the salt formed between Magnesium and Bromine? What is the chemical formula of the salt formed between Calcium and Carbon? What is the name of the salt formed between Calcium and Carbon? What is the chemical formula of the salt formed between Lithium and Phosphorus? What is the name of the salt formed between Lithium and Phosphorus? What is the chemical formula of the salt formed between Potassium and Sulfur? What is the name of the salt formed between Potassium and Sulfur? What is the chemical formula of the salt formed between Calcium and Nitrogen? What is the name of the salt formed between Calcium and Nitrogen? What is the chemical formula of the salt formed between Beryllium and Oxygen? What is the name of the salt formed between Beryllium and…arrow_forwardBALANCE the VALENCE Complete the table below. Find an element in the periodic table of elements which will completely cancel out the valence of the given element.arrow_forward
- 8. Now we will talk about when an atom is not neutral. This is called an ION. Ions occur when the protons and electrons are not equal. On the Atom Module, I want you to create Beryllium with a mass number of 9 but only add 2 electrons. HoW many electrons would need to be added if the atom was neutral? What is the charge of the ion when you only have 2 electrons? How is the charge determined? 9. Now go to the Symbol Module and create the same atom. Write down the atomic notation for the ion. 10. Using either the Atom or Symbol Modules, create an Oxygen ion that has a -2 charge and a mass number of 18. Determine the number of protons, electrons, and neutrons in the ion. 11. If you want to create a positive ion does the number of protons or electrons have to be greater? If you want to create a negative ion does the number of protons or electrons have to be greater? 12. The number of protons is always the same as the atomic number for the atom and can NEVER change. Therefore, ions are…arrow_forwardPhosphorus-32 is often used to image biological tissue. Mark any/all that apply to phosphorus-32. It has 17 protons. O It symbol is 15p It has 15 electrons. It has 17 neutrons. It symbol is 32P. It has 32 protons. It has 15 protons.arrow_forwardCount total valence electrons in: BH4 Enter the intermediate information for setting up this calculation. B Number of atoms Number of valence electrons per atom H Number of atoms Number of valence electrons per atom Electrons to form ion (enter O in each blank if substance is not an ion) Number of electrons Are these electrons added to or subtracted from the total? (Choices are: add, subtract) 7 2 #3 $ 4 15 % 6 & 7 CO 8arrow_forward
- ings ools Molecular compounds are usually composed solely of nonmetals. A binary molecular compound is one in which the compound contains only two elements (regardless of how many atoms are present of each). When naming binary molecular compounds, prefixes are used to specify the number of atoms of each element. Take a moment to review some of the prefixes shown here. Prefix Number mono di nona one three tetra four penta five hexa six hepta seven octa eight nine deca two ten For example, SF6 is named sulfur hexafluoride. Note that the prefix mono is not used in naming the first element. Also note that the second element in the name should end with the suffix ide. ▼ Part A Using the rules for naming molecular compounds described in the introduction, what is the name for the compound PC15? Spell out the full name of the compound. ►View Available Hint(s) Submit Part B Using the rules for naming molecular compounds described in the introduction, what is the name for the compound N₂ CL?…arrow_forwardCalculate M+ (using the lower mass number isotope of the halogen) and M+2 (using the higher mass number isotope of the halogen) for each of the molecular formulas given below. 1. C3H5Br 2. C6H5Cl 3. C2H3O2Cl 4. C3H4NBrarrow_forwardWrite the chemical formula of each of the acids listed in the table below. acid perchloric acid chlorous acid sulfuric acid hydrofluoric acid chemical formula ☑arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY