
A dilute aqueous solution of H2SO4 (Solution A) is to be mixed with a solution containing 90.0 wt% H2SO4 (Solution B) to produce a 75.0 wt% solution (Solution C).
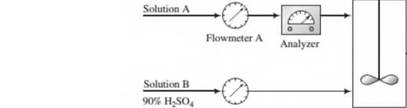
The flow rate and concentration of Solution A change periodically, so that it is necessary to adjust the flow rate of Solution B to keep the product H2SO4 concentration constant.
Flowmeters A and B have linear calibration plots of mass flow rate (m) versus meter reading (/?),
| which pass through the following points: | ||
| Flowmeter A: | mA = 150 lbn,/h, wa = 500 lbm/h. | «a = 25 «a = 70 |
| Flowmeter B: | riiB = 200 lbm/h, mB= 800 lbm/h. | Rb = 20 /?B = 60 |
The analyzer calibration is a straight line on a semilog plot of %H2SO4(x) on a logarithmic scale versus meter reading (/?,) on a linear scale. The line passes through the points (x = 20%, Rx= 4.0) and (x = 100%./?, = 10.0).
- Calculate the flow' rate of Solution B needed to process 300 lbm/h of 55% H2SO4 (Solution A), and the resulting flow rate of Solution C. (The calibration data are not needed for this part.)
- Derive the calibration equations for /ha(#a). «ib(^b), andx(/?v). Calculate the values of R\, Ru, and Rxcorresponding to the flow rates and concentrations of Part (a).
- The process technician's job is to read Flowmeter A and the analyzer periodically, and then to adjust the flow rate of Solution B to its required value. Derive a formula that the technician can use for Rbin terms of RAand Rx, and then check it by substituting the values of Part (a).
Learn your wayIncludes step-by-step video

Chapter 4 Solutions
ELEM.PRIN.OF CHEMICAL PROC.-W/ACCESS
Additional Engineering Textbook Solutions
Introduction To Programming Using Visual Basic (11th Edition)
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
Database Concepts (8th Edition)
Starting Out with Python (4th Edition)
Concepts Of Programming Languages
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
- Topic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Cash Flow AnalysisDevelop a projected cash flow statement for the first 10 years of plant operation, consideringall the costs and revenues. Include working capital, loans, and interest payments if applicable. Use following attached Process Flow Diagram as reference for this question.arrow_forwardTopic: Production of propylene glycol from glycerol derived from palm oil.QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Operational Cost AnalysisCalculate the yearly operational costs, including raw materials, labor, utilities, maintenance,and other recurring expenses. Provide a clear explanation of how these costs are derived. Use following attached Process Flow Diagram as reference for this question.arrow_forwardChemical Engineering Questionarrow_forward
- A steam boiler or steam generator is a device used to produce steam by transferring heat to water. In our case, the combustion chamber is fueled with propane (C3H8) at a flowrate of 50.0 mol/h in an excess air of 50%. Assume that both propane and air are fed at 25ºC and the combustion gases leave the chamber at 200ºC. Pressure can be assumed to be atmospheric.* Determine: 1. The heat obtained assuming complete combustion. Compare the results using elements or compounds 2. The steam flowrate that could be generated if the heat is directed to obtain superheated steam at 2 bar and 160ºC from saturated liquid water at this pressure solvearrow_forwardIn a surface coating operation, a polymer (plastic) dissolved in liquid acetone is sprayed on a solid surface and a stream of hot air is then blown over the surface, vaporizing the acetone and leaving a residual polymer film of uniform thickness. Because environmental standards do not allow discharg- ing acetone into the atmosphere, a proposal to incinerate the stream is to be evaluated. The proposed process uses two parallel columns containing beds of solid particles. The air– acetone stream, which contains acetone and oxygen in stoichiometric proportion, enters one of the beds at 1500 mm Hg absolute at a rate of 1410 standard cubic meters per minute. The particles in the bed have been preheated and transfer heat to the gas. The mixture ignites when its temperature reaches 562 C, and combustion takes place rapidly and adiabatically. The combustion products then pass through and heat the particles in the second bed, cooling down to 350 C in the process. Period- ically the flow is…arrow_forwardPropane is burned completely with excess oxygen. The product gas contains 24.5 mole% CO2, 6.10% CO, 40.8% H2O, and 28.6% O2. (a) Calculate the percentage excess O2 fed to the furnace. (b) A student wrote the stoichiometric equation of the combustion of propane to form CO2 and CO as: 2C3H8 + 11O2 → 3CO2 + 3CO + 8H2O According to this equation, CO2 and CO should be in a ratio of 1/1 in the reaction products, but in the product gas of Part (a) they are in a ratio of 24.8/6.12. Is that result possible? (Hint: Yes.) Explain howarrow_forward
- Enumerate the various methods for catalyst preparation and discuss vividly any one of the methodsarrow_forward2. Design a spherical tank, with a wall thickness of 2.5 cm that will ensure that no more than 45 kg of hydrogen will be lost per year. The tank, which will operate at 500 °C, can be made from nickel, aluminum, copper, or iron (BCC). The diffusion coefficient of hydrogen and the cost per pound for each available material is listed in Table 1. Material Do (m2/s) Q (J/mol) Cost ($/kg) Nickel 5.5 x 10-7 37.2 16.09 Aluminium 1.6 x 10-5 43.2 2.66 Copper 1.1 x 10-6 39.3 9.48 Iron (BCC) 1.2 × 10-7 15.1 0.45 Table 1: Diffusion data for hydrogen at 500 °C and the cost of material.arrow_forwardA flash drum at 1.0 atm is separating a feed consisting of methanol and water. If the feed rate is 2000 kg/h and the feed is 45 wt % methanol, what are the values of L (kg/h), V (kg/h), yM, xM (weight fractions), and Tdrum if 35% by weight of the feed is vaporized? VLE data are in Table 2-8.arrow_forward
- Q1.B. Make a comparison between current control PWM rectifier in the abc reference frame and dq reference frame.arrow_forwardstep by steparrow_forwardThe power out of an adiabatic steam turbine is 5 MW and the steam enters turbine at 2 MPa and velocity of 50 m/s, specific enthalpy (h) of 3248 kJ/kg. The elevation of the inlet is 10 m higher than at the datum. The vapor mixture exits at 15 kPa and a velocity of 180 m/s, specific enthalpy (h) of 2361.01 kJ/kg. The elevation of the exit is 6 m higher than at the datum. Let g = 9.81 m/s². Assuming the ideal gas model and R = 0.462 KJ/(kg.K). The steam specific heat ratio is 1.283. Calculate:arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





