
Concept explainers
Complete the blank cells in the following table of properties of refrigerant-134a. In the last column, describe the condition of refrigerant-134a as compressed liquid, saturated mixture, superheated vapor, or insufficient information, and, if applicable, give the quality.
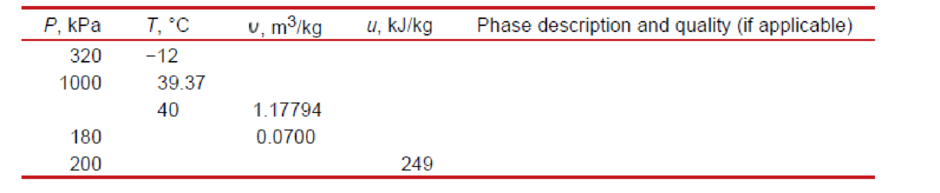
The following table for refrigerant-134a which are blank.
| P, kPa | u, kJ/kg | x, quality | Phase description | ||
| 320 | -12 | ||||
| 1000 | 39.37 | ||||
| 40 | 1.17794 | ||||
| 180 | 0.0700 | ||||
| 200 | 249 |
Explanation of Solution
State 1
Refer to Table A-12, obtain the value of saturated temperature at a pressure of 320 kPa as
The given temperature in state 1 is less than the saturated temperature at a pressure of 320 kPa.
Hence, state 1 is compressed liquid.
As wee see now there is no data for compressed liquid water in table A-7 for pressure 320 kPa, so calculate the specific internal energy and specific volume of a mixture at a saturated refrigerant-134a at a temperature of
State 2
Refer to Table A-4, obtain the specific volume at saturated liquid and specific internal energy at saturated liquid at a temperature of
Thus, the state 2 condition is saturated liquid.
State 3
Refer to Table A-13, “Superheater refrigerant-134a”, obtain the pressure and specific internal energy at a temperature and specific volume of
The given specific internal energy is greater than the specific internal energy at saturated vapour at a pressure of 140 kPa refer from Table A-12.
Thus, state 3 is a superheated steam.
State 4
Refer to Table A-12, obtain the specific volume and specific internal energy at saturated liquid
As we see now the given specific volume of the mixture
Hence, the state 4 is known as saturated mixture.
Refer to Table A-12, obtain the temperature at a pressure of 180 kPa as
Calculate the quality at state 1.
Substitute
Calculate the specific internal state.
Here, specific internal energy at saturated liquid and saturated vapour is
Substitute
State 5
Since
Thus, the state 5 is superheated steam.
Convert the unit of pressure from kPa to MPa.
Refer to Table A-13, obtain the temperature and specific volume at a pressure of 0.20 MPa and specific intenal energy of 249 kJ/kg as
From the above calculations and referred from the steam table, complete the table of
| P, kPa | u, kJ/kg | x, quality | Phase description | ||
| 320 | -12 | --- | compressed liquid | ||
| 1000 | 39.37 | -- | saturated liquid | ||
| 40 | 1.17794 | - | superheated steam | ||
| 180 | 0.0700 | saturated mixture | |||
| 200 | 249 | -- | superheated steam |
Want to see more full solutions like this?
Chapter 3 Solutions
Thermodynamics: An Engineering Approach
- Assume the xy plane is level ground, and that the vertical pole shown in the diagram lies along the z-axis with its base at the origin. If the pole is 5 m tall, and a rope is used to pull on the top of the pole with a force of 400 N as shown, determine the magnitudes of the parallel and perpendicular components of the force vector with respect to the axis of the post i.e. with respect to the z-axis.arrow_forward4-1 Q4: Q5: (20 Marks) Find √48 using False Position Method with three iterations. Hint: the root lies between 3 and 4. (20 Marks)arrow_forwardDetermine the angle between vectors FA and FB that is less than 180 degrees. FA is the vector drawn from the origin to point A (-4, 4, 2) while FB is the vector drawn from the origin to point B (3, 1, -3).arrow_forward
- Find the resultant force vector from adding F1, F2 and F3, where … F1 = {-8i+10j-32k} N F2 is 40 N in magnitude with coordinate direction angles α, β, and γ, of 45, 120 and 60 degrees, respectively and F3 is 22 N in magnitude with transverse and azimuth angles of 65 and 40 degrees, respectively Express your final answer as a Cartesian vector as well as a magnitude with angles.arrow_forwardA 2-kW resistance heater wire with thermal conductivity of k=20 W/mK, a diameter of D=4mm, and a length of L=0.9m is used to boil water. If the outer surface temp of the resistance wire is Ts=110 degrees C, determine the temp at the center of the wire.arrow_forwardA flat-plate solar collector is used to heat water by having water flow through tubes attached at the back of the thin solar absorber plate. The absorber plate has emmisssivity and an absorptivity of 0.9. The top surface where x=0 temp of the absorber is T0=35 degrees C, and solar radiation is incident on the basorber at 500 W/m^2 with a surrounding temp of 0 degrees C. The convection heat transfer coefficient at the absorber surface is 5 W/m^2 K, while the ambient temp is 25 degrees C. Show that the variation of the temp in the basorber plate can be expressed as T(x)=-(q0/k)x+T0, and determine net heat flux, q, absorbed by solar collector.arrow_forward
- Using properties of a saturated water, explain how you would determine the mole fraction of water vapor at the surface of a lake when the temp of the lake surface and the atmospheric pressure are specified.arrow_forwardConsider a glass of water in a room at 15 degrees C and 97 kPa. If the relative humidity in the room is 100 percent and the water and the air are in thermal and phase equilibrium, determine the mole fraction of the water vapor in the air and the mole fraction of air in the water.arrow_forwardStaring with an energy balance on a cylindirical shell volume element, derive the steady one dimensional heat conduction equation for a long cylinder with constant thermal conductivity in which heat is generated at a rate of egen.arrow_forward
- Consider a round potato being baked in an oven. Would you model the heat transfer to the potato as one, two, or three dimensional? Would the heat transfer be steady or transient? Also, which coordinate system would you use to solve this problem, and where would you place the origin? Explain.arrow_forward0 = 6 a = 25 t = 3 Y b = 30 xarrow_forwardSolve this problem and show all of the workarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





