
Concept explainers
It is observed that as temperature is increased, most protein molecules go from their defined, folded state into a random-coil, denatured state that exposes more hydrophobic surface area than is exposed in the folded state.
a. Given what you have learned so about
b. Sometimes, however, proteins as temperature is decreased. How might this be explained? (Hint: Consider Problem 8)
7. Assume that some protein molecule, in its folded native state, has one favored conformation. But when it is denatured, it becomes a “random coil,” with many possible conformations.
a. If we only consider the entropy for the protein, what must be the sign of
b. How will the contribution of
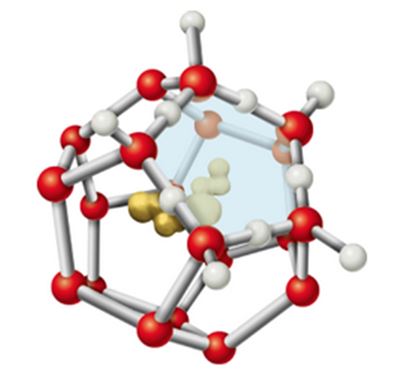
8. When a hydrophobic substance like a hydrocarbon is dissolved in water, a clathrate cage of ordered water molecules is formed about it (see Figure 2.14). If we consider only the effects on water, what do you expect the sign of

Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Biochemistry: Concepts and Connections
- Match the three types of neurotransmitters to their relative size (largest to smallest): Largest Peptide neurotransmitter ✓ Second largest [Choose] Smallest > [Choose ] [Choose ] Amino acid neurotransmitter Peptide neurotransmitter Amine neurotransmitterarrow_forwardneed help not sure why its wrong please helparrow_forwardWhy the 2nd choice is correct?arrow_forward
- a) What are the differences between the Direct & Indirect Immunofluorescence Assays? (0.5 mark) b) What are the advantages of the Indirect Immunofluorescence Assays? (0.5 mark) c) A Super-Resolution Imaging Technique was developed in 2018 using imidazole, a His-tag ligand conjugated with a fluorophore to report the presence of a recombinant His-tag protein target, (Sci Rep, 2018, 8:5507). How does this technique improve the image quality? (2 marks)arrow_forwarda) What are the differences between the Direct & Indirect Immunofluorescence Assays? b) What are the advantages of the Indirect Immunofluorescence Assays? c) A Super-Resolution Imaging Technique was developed in 2018 using imidazole, a His-tag ligand conjugated with a fluorophore to report the presence of a recombinant His-tag protein target, (Sci Rep, 2018, 8:5507). How does this technique improve the image quality?arrow_forwardCalculate the number of ATP produced from oxidation of 1 molecule of glucosearrow_forward
- Example 1: 1. Suppose an enzyme (MW = 5,000 g/mole) has a concentration of 0.05 mg/L. If the kcat is 1 x 10 s, what is the theoretical maximum reaction velocity for the enzyme? A) 1050 µM/s. B) 100 µM/s. C) 150 μM/s. D) 105 μM/s.arrow_forwardIn 1956, E. P. Kennedy and S. B. Weiss published their study of membrane lipid phosphatidylcholine (lecithin) synthesis in rat liver. Their hypothesis was that phosphocholine joined with some cellular component to yield lecithin. In an earlier experiment, incubating 32 P-labeled phosphocholine at physiological temperature (37 °C) with broken cells from rat liver yielded labeled lecithin. This became their assay for the enzymes involved in lecithin synthesis. Determine the optimal pH for this enzyme and characterize the enzyme activity at different pH values. -O-P-O-CH2-CH₁₂-N(CH3)3 Phosphocholine H₂C-O-C-R HC-O-C-R2 + + + Cell fraction + ? HC-O-P-O-CH₁₂-CH₂-N(CH), O Phosphatidylcholine The researchers then centrifuged the broken cell preparation to separate the membranes from the soluble proteins. They tested three preparations: whole extract, membranes, and soluble proteins. Table 1 summarizes the results. Table 1: Cell fraction requirement for incorporation of 32p-phosphocholine into…arrow_forwardResearchers isolated an unknown substance, X, from rabbit muscle. They determined its structure from the following observations and experiments. (a) Qualitative analysis showed that X was composed entirely of C, H, and O. A weighed sample of X was completely oxidized and the H2O and CO2 produced were measured. This quantitative analysis revealed that X contained 40.00% C, 6.71% H, and 53.29% O by weight. (b) The molecular mass of X, as determined by mass spectrometry, was 90.00 atomic mass units (u). (c) Infrared spectroscopy showed that X contained one double bond. (d) X dissolved readily in water, and the solution demonstrated optical activity when tested in a polarimeter. (e) The aqueous solution of X is acidic. What is the empirical formula of X?arrow_forward
- Show work. don't give Ai generated solution....give correct solutionarrow_forwardBiochemistry What is the process of "transamination" in either the muscles or the liver, that involves keto acid or glutamic acid? Please explain how the steps work. Thank you!arrow_forwardBiochemistry Please help. Thank you What is the importance of glutamic acid in the metabolism of nitrogen from amino acids? (we know therole; it’s used to remove the nitrogen from amino acids so that the remaining carbon skeleton can bebroken down by the “usual” pathways, but what is the important, unique role that only glutamicacid/glutamate can do?)arrow_forward
 Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax





