
Quinapril (trade name Accupril) is a drug used to treat hypertension and congestive heart
failure.
a. Identify the
b. Classify any alcohol, amide or
c. At which sites can quinapril hydrogen bond to water?
d. At which sites can quinapril hydrogen bond to acetone
e. Label the most acidic hydrogen atom.
f. Which site is most basic?
(a)
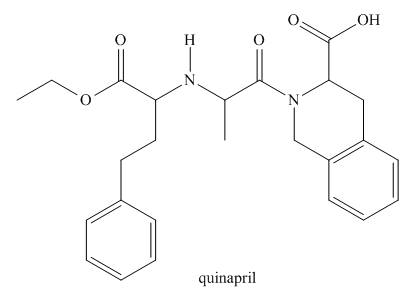
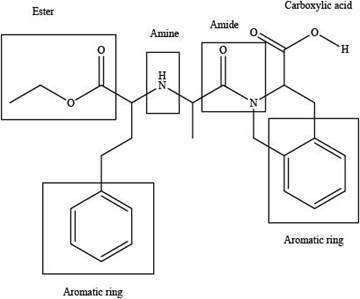
Interpretation: The functional groups present in the given compound, quinapril are to be identified.
Concept introduction: An atom or a group of atoms that shows characteristic physical and chemical properties are collectively known as functional groups. The functional group is the most reactive part present in the molecule. The main functional groups are
Answer to Problem 3.60P
The functional groups present in the given compound, quinapril are shown below.
Explanation of Solution
The functional groups present in the given compound, quinapril are shown as,
Figure 1
Thus, there are total four functional groups present in quinapril which are ester group, amine group, amide group and carboxylic group as highlighted above. There are two aromatic also present in the given compound, quinapril.
There are total four functional groups present in quinapril which are ester group, amine group, amide group and carboxylic group.
(b)
Interpretation: The alcohol, amine or amide groups present in the given compound, quinapril are to be classified as
Concept introduction: The amine, amide or alcoholic groups are identified as primary
Answer to Problem 3.60P
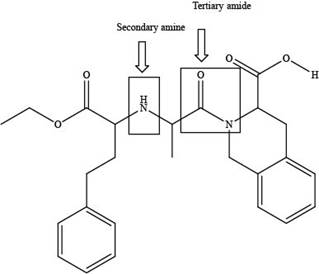
The classification of alcohol, amine or amide groups present in the given compound, quinapril as
Explanation of Solution
The classification alcohol, amine or amide groups present in the given compound, quinapril are shown as,
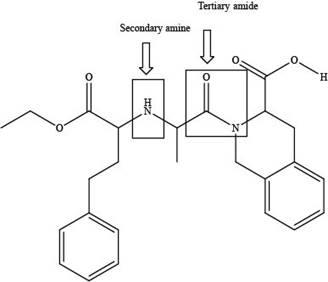
Figure 2
The given compound consists of one tertiary amide and one secondary amine as shown above.
The classification of alcohol, amine or amide groups present in the given compound, quinapril as
(c)
Interpretation: The site at which quinapril forms hydrogen bond with water is to be predicted.
Concept introduction: The bonding that takes place between hydrogen atom and hetero atoms like oxygen, nitrogen and fluorine is known as hydrogen bonding. In case of water, hydrogen atom makes bond with each other. Hydrogen bonding is the strongest bonding among the intermolecular forces and covalent bonding.
Answer to Problem 3.60P
The site at which quinapril forms hydrogen bond with water is shown below.
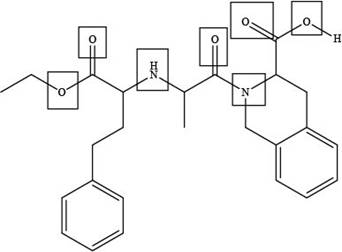
Explanation of Solution
The site at which quinapril forms hydrogen bond with water is shown as,

Figure 3
In the given compound, the sites where the electronegative atoms like, oxygen and nitrogen are present, there only hydrogen bond forms with water as highlighted above.
The site at which quinapril forms hydrogen bond with water is shown in Figure 3.
(d)
Interpretation: The site at which quinapril forms hydrogen bond with acetone is to be predicted.
Concept introduction: The bonding that takes place between hydrogen atom and hetero atoms like oxygen, nitrogen and fluorine is known as hydrogen bonding. In case of acetone, hydrogen atom makes bond with that electronegative atom which is attached to hydrogen atom only. Hydrogen bonding is the strongest bonding among the intermolecular forces and covalent bonding.
Answer to Problem 3.60P
The site at which quinapril forms hydrogen bond with acetone is shown below.
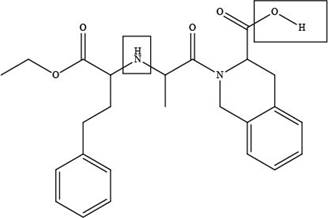
Explanation of Solution
The site at which quinapril forms hydrogen bond with acetone is shown as,

Figure 4
In the given compound, the sites where the electronegative atoms like, oxygen and nitrogen attached to other hydrogen atom are present, there only hydrogen bond forms with acetone as highlighted above.
The site at which quinapril forms hydrogen bond with acetone is shown in Figure 4.
(e)
Interpretation: The most acidic hydrogen present in the given compound, quinapril is to be labeled.
Concept introduction: The most acidic hydrogen
Answer to Problem 3.60P
The labeling of the most acidic hydrogen present in the given compound, quinapril is shown below.
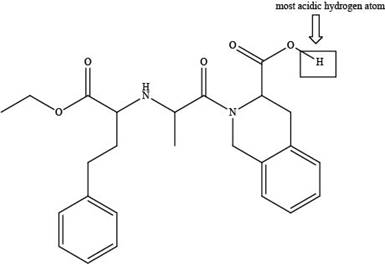
Explanation of Solution
The labeling of the most acidic hydrogen present in the given compound, quinapril is shown as,

Figure 5
The electronegativity increases while moving left to right in the period. Thus, oxygen is more electronegative than nitrogen atom as it is located at the extreme right position. According to the structure of quinapril, hydrogen atom that is bonded with oxygen atom is the most acidic one because oxygen is more electronegative than nitrogen atom. The higher electronegativity corresponds to the greater acidic character of hydrogen atom attached to the electronegative atom.
The labeling of the most acidic hydrogen present in the given compound, quinapril is shown in Figure 5.
(f)
Interpretation: The most basic site in the compound quinapril is to be labeled.
Concept introduction: According to Bronsted-Lowry theory, the species that easily tends to accept the proton is known as base and the species that easily donate the proton is known as acid. The high delocalization of electron pair corresponds to the high basic character of the atom.
Answer to Problem 3.60P
The labeling of the most basic site in the compound quinapril is shown below.
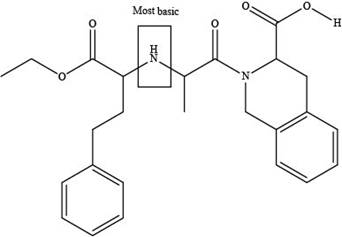
Explanation of Solution
The labeling of the most basic site in the compound quinapril is shown as,
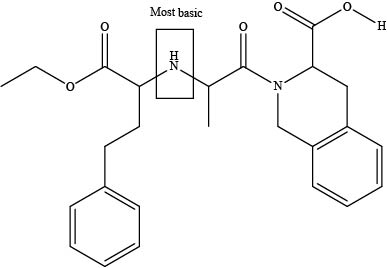
Figure 6
According to the structure of quinapril, the site at which amide group is present is the most basic site because in amides, the pair of electrons over nitrogen atom gets highly delocalized. Hence, the most basic site is present at tertiary amide.
The labeling of the most basic site in the compound quinapril is shown in Figure 6.
Want to see more full solutions like this?
Chapter 3 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- The number of imaginary replicas of a system of N particlesA) can never become infiniteB) can become infiniteC) cannot be greater than Avogadro's numberD) is always greater than Avogadro's number.arrow_forwardElectronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Calculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forwardGeneral formula etherarrow_forwardPlease provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote! Please correct answer and don't used hand raitingarrow_forward
- Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forward(please correct answer and don't used hand raiting) Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forwardCaTiO3 has a perovskite structure. Calculate the packing factor.Data: ionic radii Co+2 = 0.106 nm, Ti+4 = 0.064 nm, O-2 = 0.132 nm; lattice constant is a = 2(rTi4+ + rO-2).(a) 0.581(b) -0.581(c) 0.254(d) -0.254arrow_forward
- In the initial linear section of the stress-strain curve of a metal or alloy. Explain from the point of view of atomic structure?(a) No, the atomic level properties of the material can never be related to the linear section.(b) The elastic zone is influenced by the strength of the bonds between atoms.(c) The stronger the bond, the less rigid and the lower the Young's Modulus of the material tested.(d) The stronger the bond, the less stress is necessary to apply to the material to deform it elastically.arrow_forwardThe degree of polymerization of polytetrafluoroethylene (Teflon) is 7500 (mers/mol). If all polymer chains have equal length, state the molecular weight of the polymer and the total number of chains in 1000 g of the polymer(a) 50 000 g/mol; 0.03·1020 chains(b) 100 000 g/mol; 1.03·1020 chains(c) 750 000 g/mol; 8.03·1020 chainsarrow_forwardIn natural rubber or polyisoprene, the trans isomer leads to a higher degree of crystallinity and density than the cis isomer of the same polymer, because(a) it is more symmetrical and regular.(b) it is less symmetrical.(c) it is irregular.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

