
Identify each
represents a lactone-a cyclic ester- and which represents a lactam-a cyclic amide?
a. 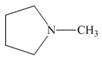 b.
b. 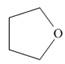 c.
c. 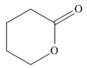 d.
d. 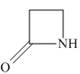
(a)
Interpretation: The functional group present in the given ring and the structure that represent a lactone and a lactam is to be stated.
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester |
Answer to Problem 3.33P
The functional group present in the given ring is amine. The given structure neither represents a lactone nor a lactam.
Explanation of Solution
The given structure is,
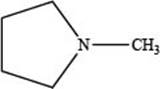
Figure 1
The given structure contains amine as a functional group. In the given structure, nitrogen is bonded to three carbon atoms. Therefore, the given ring is a tertiary amine.
Lactone is a cyclic ester, whereas lactam is a cyclic amide. Thus, the given structure neither represents a lactone nor a lactam.
The functional group present in the given ring is amine. The given structure neither represents a lactone nor a lactam.
(b)
Interpretation: The functional group present in the given ring and the structure that represent a lactone-cyclic ester and a lactam-a cyclic amide is to be stated.
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester |
Answer to Problem 3.33P
The functional group present in the given ring is ether. The given structure neither represents a lactone nor a lactam.
Explanation of Solution
The given structure is,
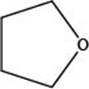
Figure 2
The given structure contains ether as a functional group. The given functional group is in cyclic form. Therefore, the given structure is cyclic ether. Lactone is a cyclic ester, whereas lactam is a cyclic amide. Thus, the given structure neither represents a lactone nor a lactam.
The functional group present in the given ring is ether. The given structure neither represents a lactone nor a lactam.
(c)
Interpretation: The functional group present in the given ring and the structure that represent a lactone-cyclic ester and a lactam-a cyclic amide is to be stated.
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester |
Answer to Problem 3.33P
The functional group present in the given ring is ester. The given structure represents a lactone-cyclic ester.
Explanation of Solution
The given structure is,
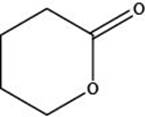
Figure 3
In the given structure, the functional group present is ester. The given functional group is in cyclic form. Therefore, the given structure represents a lactone-cyclic ester.
The functional group present in the given ring is ester. The given structure represents a lactone-cyclic ester.
(d)
Interpretation: The functional group present in the given ring and the structure that represent a lactone-cyclic ester and a lactam-a cyclic amide is to be stated.
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester |
Answer to Problem 3.33P
The functional group present in the given ring is amide. The given structure represents a lactam-a cyclic amide.
Explanation of Solution
The given structure is,
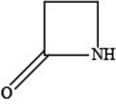
Figure 4
In the given structure, the functional group present is amide. The given functional group is in cyclic form. Therefore, the given structure represents a lactam-a cyclic amide.
The functional group present in the given ring is amide. The given structure represents a lactam-a cyclic amide.
Want to see more full solutions like this?
Chapter 3 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Amorphous polymers are usually transparent and semi-crystalline polymers are usually opaque. Correct?(a) No. They are all made up of polymer chains. True if they were monomers.(b) Yes. The arrangement of the chains determines the passage of light.(c) No. It is the other way around.(d) Crystallinity or amorphousness does not affect the transparency or opacity of the material.arrow_forwardThe name ferrites refers to a family of(a) ceramic materials that exhibit ferrimagnetic behavior due to their ionic composition.(b) polymeric materials that exhibit ferrimagnetic behavior due to their ionic composition.(c) concrete-based materials that exhibit ferrimagnetic behavior due to their ionic composition.(d) superconducting materials that exhibit ferrimagnetic behavior due to their ionic composition.arrow_forwardState the two main factors affecting ion packing in the solid state.(a) Number of covalent bonds and their unsaturation.(b) Mechanical properties and degradation temperature.(c) Number of crystalline phases present and grain size.(d) Electroneutrality and ion size.arrow_forward
- The ceramic materials alumina (Al2O3) and chromium oxide (Cr2O3) form an isomorphic phase diagram. The solubility will be(a) unlimited of one ceramic in the other.(b) very limited depending on the weight % of Al2O3(c) very limited depending on the weight % of Cr2O3(d) partial of one ceramic in the other.arrow_forwardAmong the main characteristics of optical fibers, indicate which of the following is not included:(a) Opacity and Rigidity(b) Flexibility(c) Transparency(d) Low thicknessarrow_forwardMost ceramic materials have low thermal conductivities because(a) Electron mobility is strongly restricted due to their strong ionic-covalent bonding.(b) False, in general they are excellent thermal conductors (they are used in ovens).(c) Electron mobility is dependent on T and therefore they are poor conductors at high temperatures.(d) Electron mobility is highly restricted by secondary bonds.arrow_forward
- Si increases its conductivity when doped with Ga and P.(a) True, because the conduction mechanism is due to electrons and holes generated by Ga and P as the case may be.(b) True, because a completely different compound is generated.(c) False, because when impurities are introduced, the opposite occurs.(d) False, because the conductivity of Si is only determined by the increase in temperature, which must be controlled.arrow_forwardIndicate whether a configuration and a microstate are the same:a) Yesb) No, a microstate encompasses several configurationsc) No, a configuration is the same as a macrostated) No, a configuration encompasses several microstatesarrow_forwardThe representation of a one-dimensional velocity distribution function for a gas, with increasing temperature the maximum occurs for vi = 0 m/s. Correct?arrow_forward
- The representation of a one-dimensional velocity distribution function for a gas, as the temperature increases:a) it becomes more flattenedb) the maximum occurs for vi = 0 m/sExplain it.arrow_forwardThe velocity distribution function of gas moleculesa) is used to measure their velocity, since the small size of gas molecules means that it cannot be measured in any other wayb) is only used to describe the velocity of particles if their density is very high.c) describes the probability that a gas particle has a velocity in a given interval of velocitiesarrow_forwardExplain why in the representation of a one-dimensional velocity distribution function for a particular gas, the maximum occurs for vi = 0 m/s.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





