
Draw the structure of a compound fitting each description:
a. an
b. a
c. a
d. an ester with molecular formula
(a)
Interpretation: The structure of an aldehyde with molecular formula
Concept introduction: Functional groups are specific substituents present in the molecule that is responsible for the characteristic chemical reactions. For example, functional groups possess a carbonyl group includes aldehydes, ketones, carboxylic acids, amides, esters and acid chlorides. An organic compound containing
Answer to Problem 3.10P
The structure of an aldehyde with molecular formula
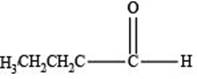
Explanation of Solution
The given molecular formula is
Where,
• C is the number of carbon atoms.
• H is the number of hydrogen atoms.
Substitute the values of number of carbon atoms and hydrogen atoms in the above formula to calculate the number of double bond in compound
Therefore, the given compound has one double bond with an aldehydic functional group. Hence, the structure an aldehyde with molecular formula
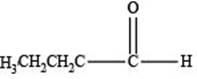
Figure 1
The structure of an aldehyde with molecular formula
(b)
Interpretation: The structure of a ketone with molecular formula
Concept introduction: Functional groups are specific substituents present in the molecule that is responsible for the characteristic chemical reactions. For example, functional groups possess a carbonyl group includes aldehydes, ketones, carboxylic acids, amides, esters and acid chlorides. An organic compound containing
Answer to Problem 3.10P
The structure of a ketone with molecular formula
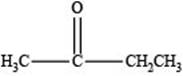
Explanation of Solution
The given molecular formula is
Where,
• C is the number of carbon atoms.
• H is the number of hydrogen atoms.
Substitute the values of number of carbon atoms and hydrogen atoms in the above formula to calculate the number of double bond in compound
Therefore, the given compound has one double bond with a ketone functional group. Hence, the structure a ketone with molecular formula

Figure 2
The structure of a ketone with molecular formula
(c)
Interpretation: The structure of a carboxylic acid with molecular formula
Concept introduction: Functional groups are specific substituents present in the molecule that is responsible for the characteristic chemical reactions. For example, functional groups possess a carbonyl group includes aldehydes, ketones, carboxylic acids, amides, esters and acid chlorides. An organic compound containing
Answer to Problem 3.10P
The structure of a carboxylic acid with molecular formula
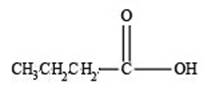
Explanation of Solution
The given molecular formula is
Where,
• C is the number of carbon atoms.
• H is the number of hydrogen atoms.
Substitute the values of number of carbon atoms and hydrogen atoms in the above formula to calculate the number of double bond in compound
Therefore, the given compound has one double bond with a carboxylic acid functional group. Hence, the structure a carboxylic acid with molecular formula
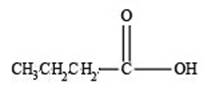
Figure 3
The structure of a carboxylic acid with molecular formula
(d)
Interpretation: The structure of an ester with molecular formula
Concept introduction: Functional groups are specific substituents present in the molecule that is responsible for the characteristic chemical reactions. For example, functional groups possess a carbonyl group includes aldehydes, ketones, carboxylic acids, amides, esters and acid chlorides. An organic compound containing
Answer to Problem 3.10P
The structure of an ester with molecular formula
Explanation of Solution
The given molecular formula is
Where,
• C is the number of carbon atoms.
• H is the number of hydrogen atoms.
Substitute the values of number of carbon atoms and hydrogen atoms in the above formula to calculate the number of double bond in compound
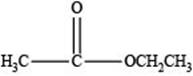
Therefore, the given compound has one double bond with an ester functional group. Hence, the structure an ester with molecular formula

Figure 4
The structure of an ester with molecular formula
Want to see more full solutions like this?
Chapter 3 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Can the target compound be efficiently synthesized in good yield from the substituted benzene of the starting material? If yes, draw the synthesis. Include all steps and all reactants.arrow_forwardThis is a synthesis question. Why is this method wrong or worse than the "correct" method? You could do it thiss way, couldn't you?arrow_forwardTry: Draw the best Lewis structure showing all non-bonding electrons and all formal charges if any: (CH3)3CCNO NCO- HN3 [CH3OH2]*arrow_forward
- What are the major products of the following reaction? Draw all the major products. If there are no major products, then there is no reaction that will take place. Use wedge and dash bonds when necessary.arrow_forwardZeolites. State their composition and structure. Give an example.arrow_forwardDon't used hand raiting and show all reactionsarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardIX) By writing the appropriate electron configurations and orbital box diagrams briefly EXPLAIN in your own words each one of the following questions: a) The bond length of the Br2 molecule is 2.28 Å, while the bond length of the compound KBr is 3.34 Å. The radius of K✶ is 1.52 Å. Determine the atomic radius in Å of the bromine atom and of the bromide ion. Br = Br b) Explain why there is a large difference in the atomic sizes or radius of the two (Br and Br). Tarrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forward
- When 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol. Which experimental number must be initialled by the Lab TA for the first run of Part 1 of the experiment? a) the heat capacity of the calorimeter b) Mass of sample c) Ti d) The molarity of the HCl e) Tfarrow_forwardPredict products for the Following organic rxn/s by writing the structurels of the correct products. Write above the line provided" your answer D2 ①CH3(CH2) 5 CH3 + D₂ (adequate)" + 2 mited) 19 Spark Spark por every item. 4 CH 3 11 3 CH 3 (CH2) 4 C-H + CH3OH CH2 CH3 + CH3 CH2OH 0 CH3 fou + KMnDy→ C43 + 2 KMn Dy→→ C-OH ") 0 C-OH 1110 (4.) 9+3 =C CH3 + HNO 3 0 + Heat> + CH3 C-OH + Heat CH2CH3 - 3 2 + D Heat H 3 CH 3 CH₂ CH₂ C = CH + 2 H₂ → 2 2arrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





