
Determine the number of unpaired electrons in each of the following atoms in the ground state and identify each as diamagnetic or paramagnetic: (a) C, (b) S, (c) Cu, (d) Pb, (e) Ti.
(a)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behaviour should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.104QP
The number of unpaired electrons in
Explanation of Solution
Carbon (
The noble gas core for
Put all the electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
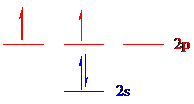
The 4 electrons of carbon (
The unpaired electrons are present in
(b)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behavior should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.104QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
Put all the electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
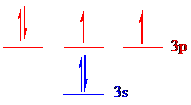
All the 6 electrons of
The unpaired electrons are present in
(c)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behavior should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.104QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
Put all the 11 electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
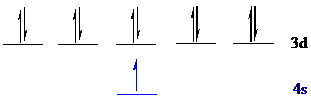
All the 11 electrons of
The unpaired electron is present in
(d)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behavior should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.104QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
Put all the 28 electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
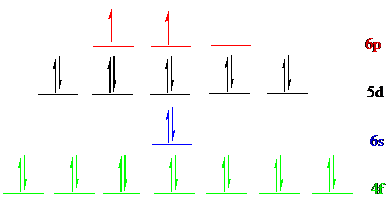
All the 28 electrons of
The unpaired electrons are present in
(e)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behavior should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.104QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
Put all the electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
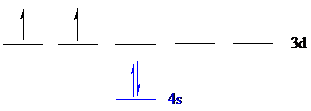
The four electrons of
The unpaired electrons are present in
Want to see more full solutions like this?
Chapter 3 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
- In the reaction of naphthalene with CrO3 in acetic acid. Indicate whether a different product is obtained if carried out at 25°C or with heating (A).arrow_forwardQUESTION: Fill in the answers in the empty green boxes 1. Step 2 2. Step 3 3. Step 4 (SUM) 4. Step 5 (df) (GIVEN) 5. Determine S y/x value *The data values have been provided in the worksheet attached in the first image*arrow_forwardIf the symbol A is placed in a reaction, at what temperature does it take place?arrow_forward
- By malonic or acetylacetic synthesis, synthesize 3-methyl-4-oxopentanoic acid (indicate the formulas of the compounds).arrow_forwardoalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.arrow_forwardWrite the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcoholarrow_forward
- What type of interaction would you expect between the following R groups in the tertiary structure of a protein? O -CH2-CO and -CH2-CH2-CH2-CH2-NH3+ a. disulfide bonds b. salt bridges c. hydrogen bonds HO abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT d. hydrophobic interactions e. peptide bondsarrow_forward4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forwardBy malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forwardDraw the formula for 3-chlorobenzoic acetic anhydride.arrow_forwardBy malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forward

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





