
Determine the number of impaired electrons in each of the following atoms in the ground state and identify each as diamagnetic or paramagnetic: (a) Rb, (b) As, (c) I, (d) Cr, (e) Zn.
(a)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behaviour should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.103QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
All the electrons are placed in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
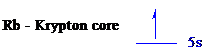
The one electron of
The unpaired electron is present in
(b)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behaviour should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.103QP
The number of unpaired electron in
Explanation of Solution
Arsenic (
The noble gas core for
Put all the 15 electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
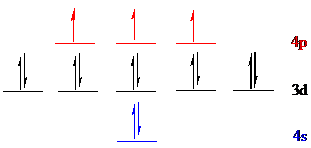
All the 15 electrons of arsenic (
The unpaired electrons are present in
(c)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behaviour should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.103QP
The number of unpaired electron in
Explanation of Solution
Iodine (
The noble gas core for
Put all the 17 electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
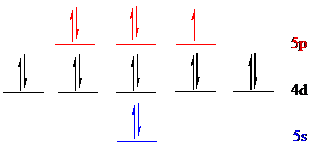
All the 17 electrons of iodine (
The unpaired electron is present in
(d)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behaviour should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.103QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
Put all the electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule
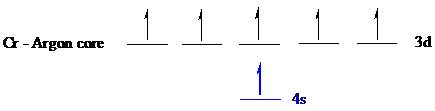
The six electrons of
The unpaired electrons are present in
(e)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behaviour should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.103QP
There is no unpaired electron in
Explanation of Solution
The noble gas core for
Put all the 12 electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
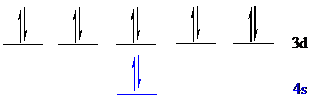
All the 12 electrons of
There is no unpaired electron present in
Want to see more full solutions like this?
Chapter 3 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
- Write the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcoholarrow_forwardWhat type of interaction would you expect between the following R groups in the tertiary structure of a protein? O -CH2-CO and -CH2-CH2-CH2-CH2-NH3+ a. disulfide bonds b. salt bridges c. hydrogen bonds HO abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT d. hydrophobic interactions e. peptide bondsarrow_forward4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forward
- By malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forwardDraw the formula for 3-chlorobenzoic acetic anhydride.arrow_forward
- By malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forwardObtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forwardEFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forward
- If we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.arrow_forwardIf we have two compounds: acetone (CH3COCH3) and acetic acid (CH3COOH); if we apply heat (A), what product(s) are obtained?arrow_forwardQUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





