
Concept explainers
a)
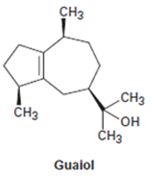
Interpretation:
The conformation of either geranyl diphosphate or farnesyl diphosphate which shows likeness to guaiol is to be drawn.
Concept introduction:
Monoterpenes containing 10 carbon atoms are derived from geranyl diphosphate while sesqueterpenes containing 15 carbon atoms are derived from farnesyl diphosphate.
To give:
The conformation of either geranyl diphosphate or farnesyl diphosphate which shows likeness to guaiol.
b)
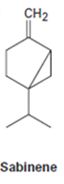
Interpretation:
The conformation of either geranyl diphosphate or farnesyl diphosphate which shows likeness to sabinene is to be drawn.
Concept introduction:
Monoterpenes containing 10 carbon atoms are derived from geranyl diphosphate while sesqueterpenes containing 15 carbon atoms are derived from farnesyl diphosphate.
To give:
The conformation of either geranyl diphosphate or farnesyl diphosphate which shows likeness to sabinene.
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
Organic Chemistry

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



